Chapter: 11th Microbiology : Chapter 14 : Microbial Genetics
DNA Structure
DNA Structure
·
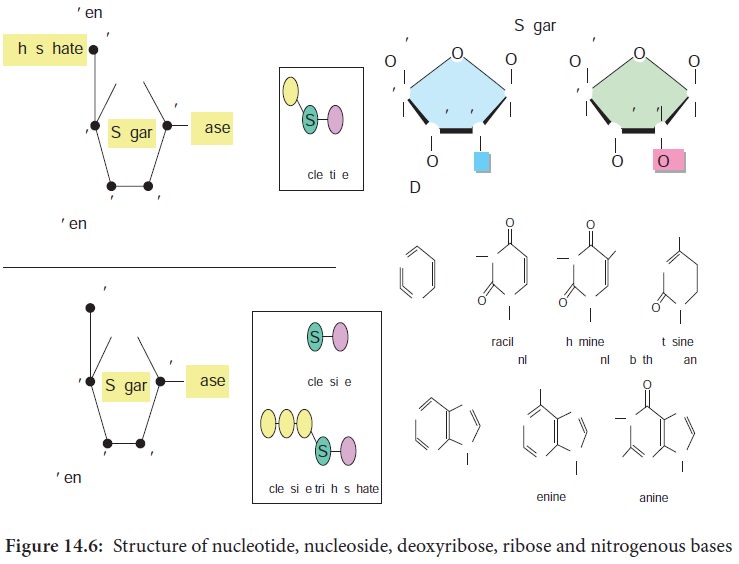
DNA is a polymer of simple monomeric units, the nucleotides
(Figure 14.6).
·
Each nucleotide is made up of three components:
1. Nitrogenous base
2. Sugar
3. Phosphate group
·
Nucleotide without phosphate group is known as nucleoside.
·
The sugar present in DNA is deoxyribose sugar.
·
The nitrogenous bases present in DNA are
Purines – Adenine (A), Guanine (G)
Pyrimidines
– Thymine(T), Cytosine (C)
·
The nucleotide as a unit is formed by
Glycosidic bond between nitrogenous base and sugar,
Ester bond between phosphate group and sugar
·
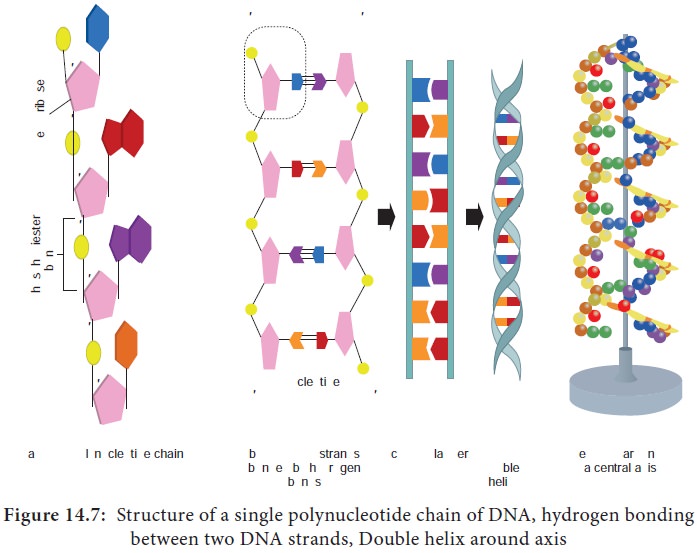
Each of the nucleotides is bonded by a phosphodiester bond to
form a polynucleotide chain (strand) (Figure 14.7a).
·
Two polynucleotide chains join together through hydrogen bonds
between nitrogenous bases, to form double stranded DNA (Figure 14.7b).
·
Two hydrogen bonds exists between adenine and thymine and three
hydrogen bonds between guanine and cytosine.
·
DNA is coiled in the form of a double helix, in which both the
strands of DNA coil around an axis (Figure 14.7d & e).
·
The further coiling of this axis upon itself produces DNA
supercoiling an important property of DNA structure.
All DNA, whether large or small, possess the same sugar
phosphate backbone. What distinguishes one DNA from another is the length of
the polymer and distribution of four bases along the backbone. The variety of
sequences that can be made from the four nitrogenous bases is limitless, as is
the number of melodies possible with a few musical notes. RNA differs from DNA
by having a ribose sugar instead of deoxyribose and nitrogenous base Uracil
instead of Thymine.
Watson And Crick Model of DNA Double Helix
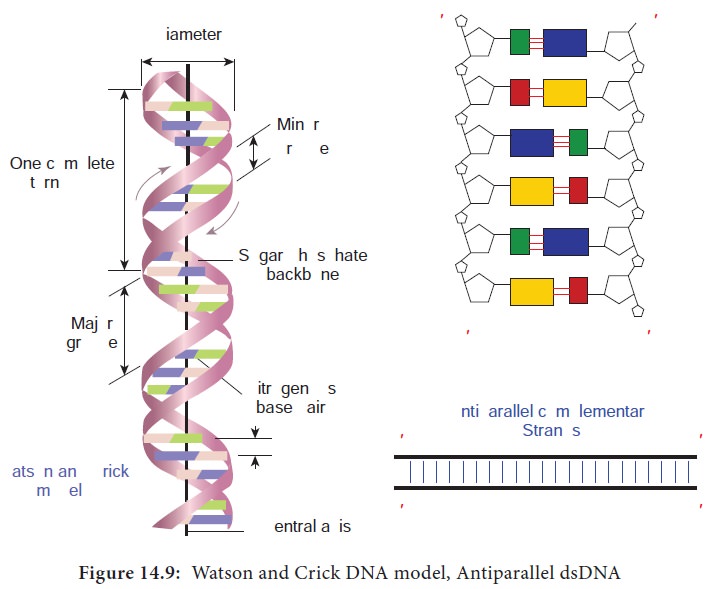
In the early 1950’s, Rosalind Franklin and Maurice Wilkins used the powerful method of X-ray diffraction to shed more light on the structure of DNA. From the X-ray Diffraction pattern it was deduced that DNA molecules are helical. In 1953 Watson and Crick (Figure 14.8) postulated a three dimensional model of DNA structure based on Franklin’s X-ray crystallographic studies. In recognition of their work leading to the double helix model, Nobel prize was awarded in 1962 to Watson, Crick and Wilkins. According to Watson and Crick model (Figure 14.9),

·
The DNA consists of two helical polynucleotide chains wound
around the same axis to form a right handed helix.
· The Purine and Pyrimidine bases of both strands are stacked inside the double helix.
·
Each nitrogenous base of one strand is paired in the same plane
with a base of the other strand.
·
According to Watson and Crick rule Adenine base pairs with
Thymine and Guanine base pairs with Cytosine.
· Two hydrogen bonds are present between A and T (symbolised as A=T) and three hydrogen bonds are present between G and C (G=C).
·
The hydrogen bonds provide chemical stability essential to hold
the two chains together.
· The specific A equal to T and G equal to C base pairing is the basis for the complementarity concept. This complementarity concept is very important in the process of DNA replication and gene expression.
·
The pairing of two strands creates a major groove and minor
groove on the surface of the duplex.
·
The two strands are antiparallel, that is their 5ʹ, 3ʹ
phosphodiester bonds run in opposite directions.
·
The vertically stacked bases are 3.4 Aº apart.
·
Each complete turn of double helix con-tain base pairs, which is
34 Aº units long.
Erwin Chargaff ’s Rule
In the late 1940s Erwin Chargaff and his colleagues found that
the four nucleotide bases of DNA occur in different ratios in the DNA of
different organisms. Erwin Chargaff measured the quantity of the bases in DNA
and noticed that the number of Adenine is equal to the number of Thymine and
the number of Guanine is equal to the number of Cytosine residues. Hence the
sum of Purine residues equal to the sum of the Pyrimidine residues.
Quantitatively A=T or A/T = 1
C=G or C/G = 1
A+G = T+C
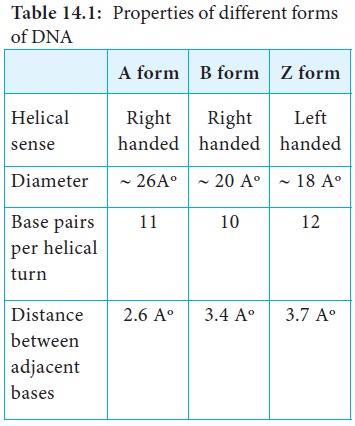
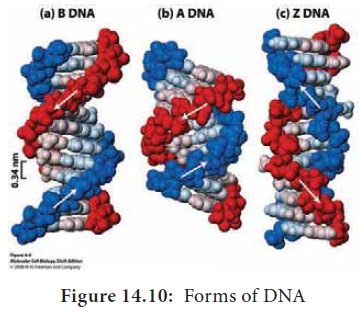
Alternative Forms of DNA
DNA is a remarkably flexible molecule. Considerable rotation is
possible around a number of bonds in sugar-phosphate backbone and thermal
fluctuations can produce bending, stretching and unpairing of the strands.
Watson and Crick model of DNA is called as B-DNA or B-form. However DNA can
exist in A or Z form. In 1979 Alexander Rich discovered Z form (Figure 14.10).
Recently, several alternative forms of DNA have been discovered C-form, D-form
and E-form. The B-form of DNA is the most stable structure and is therefore the
standard point of reference in any study of the properties of DNA (Table 14.1).
Related Topics