Chapter: Physics : Crystal Physics
Crystalllography
Crystallography
Solids are of classified into two categories
based on the arrangement o atoms or molecules.
· Crystalline Solids
· Non Crystalline Solids
or Amorphous Solids
A substance is said to be crystalline when the
arrangement of the units of the matter inside it is regular or periodic or in
an orderly fashion.
The crystalline solids may be classified into
two types based on the arrangements of the crystal in the structure.
· Single Crystal: the entire solid
consists of only one crystal.
· Poly Crystal: it has an aggregate of
many small crystals that are separated by well-defined boundaries.
In amorphous solids, there is no order in the
arrangement o their constituent particles.
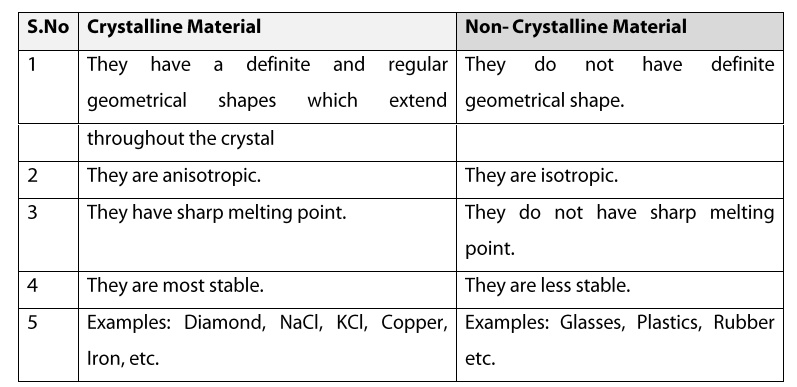
Crystalline Material
1. They have a definite and regular
geometrical shapes which extend throughout the crystal.
2. They are anisotropic.
3. They have sharp melting point.
4. They are most stable.
5. Example: Diamond, NaCl, KCl, Copper, Iron,
etc.
Non Crystalline Material
1. They do not have definite geometrical
shape.
2. They are isotropic.
3. They do not have sharp melting point.
4. Examples: Glasses, Plastics, Rubber etc.
Crystal is a regular polyhedral form bounded
by surfaces, which is formed by chemical compound under the action of its inter
atomic forces, when passing rom the state of the liquid (or) gas to that of a
solid, under suitable conditions.
The phase change from liquid (or) gas to solid
is called Crystallization.
The crystal structure gives the arrangement of
the atoms with in a crystal.
Fundamental Crystallographic terms
Lattice is defined as an array
of points which are imaginarily kept to represent the position of atoms in the
crystal that every lattice point has got the same environment as that o the
other and hence one lattice point cannot be distinguished from the other lattice
point.
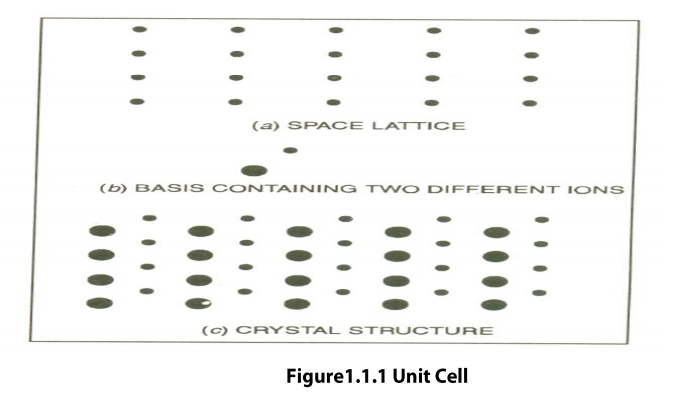
Space Lattice or Crystal Lattice is a three dimensional
collection of points in space. The environment about any particular point is in
everyway the same.
The regular pattern of points which describe
the three dimensional arrangement of particles in a crystal structure is called
the Crystal Lattice or Space lattice.
Basis is formed by associating with every
lattice point a uint of assembly of atoms or molecules identical in
composition. This unit assembly is called Basis.
The Crystal Structure is formed by the
addition of a basis to every lattice point.
Space Lattice + Basis = Crystal Structure
Unit Cell is defined as the
smallest geometric figure or pattern, the repetition of which gives the actual
crystal structure. This is also defined as undamental elementary pattern o
minimum of atoms or molecules or groups of molecules, which represents total
characteristics of the material or the crystal.
Related Topics