Chapter: Mechanical : Heat and Mass Transfer : Mass Transfer
Convective Mass Transfer
CONVECTIVE MASS TRANSFER :
(i) Diffusion in Gases - the diffusion rates in gases are dependent on the molecular speed which is a function of temperature and therefore, the diffusion coefficient depends upon the temperature of gases.
Gilliland has proposed a semi-empirical equation for diffusion coefficient in a binary gas mixture –
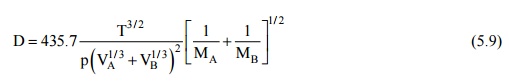
where D is in square centimeters per second, T is in Kelvin, p is the total pressure of the system in pascals, VA and VB are the molecular volumes of the species A and R as calculated from the atomic volumes in Table 12.1, MA and MB are the molecular weights of species A and B.
Diffusion coefficients for gases depend upon pressure, temperature and other molecular properties of diffusing gases. At two different pressure and temperature, we have
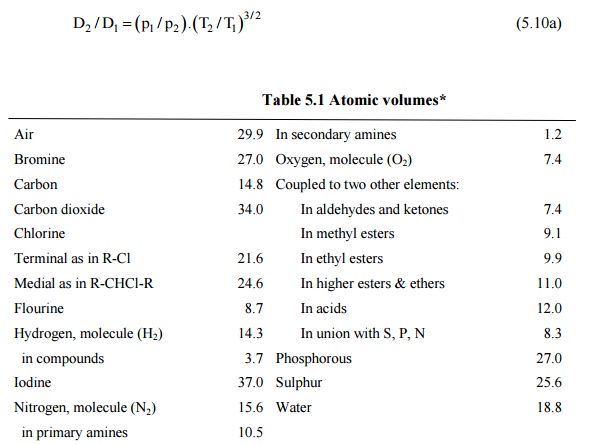
Table 5.1 Atomic volumes*
Air 29.9 In secondary amines 1.2
Bromine 27.0 Oxygen, molecule (O2) 7.4
Carbon 14.8 Coupled to two other elements:
Carbon dioxide 34.0 In aldehydes and ketones 7.4
Chlorine In methyl esters 9.1
Terminal as in R-Cl 21.6 In ethyl esters 9.9
Medial as in R-CHCl-R 24.6 In higher esters & ethers 11.0
Flourine 8.7 In acids 12.0
Hydrogen, molecule (H2) 14.3 In union with S, P, N 8.3
in compounds 3.7 Phosphorous 27.0
Iodine 37.0 Sulphur 25.6
Nitrogen, molecule (N2) 15.6 Water 18.8
in primary amines 10.5
*(For three numbered ring like ethylene oxide, deduct 6.0, for four numbered ring like cyclobutane, deduct 8.5, for six numbered ring like benzene, deduct 15.6, for napthelene ring, deduct 30.0.)
(ii) Diffusion in Liquids and Solids - Diffusion in liquids occurs at much slower rate than in gases. Since kinetic theory of liquids is not as much developed as that of gases, it is usually assumed as a first approximation that equations of the same general form are applicable to the diffusion of a solute in a solvel1t as to the diffusion in gases, i.e., Fick's law is assumed valid for liquids.
Diffusion coefficient for most of the common organic and inorganic materials in the
usual solvents such as water, alcohol and benzene at room temperature lie m the range of 1.79 × 10-3 to 1.075 × 10-7 cm2/s.
Diffusion in solids is much slower than in liquids. Diffusion of solids in solid has limited engineering applications but diffusion of fluids in solids have extensive applications. Fick's law is sometimes used, with an empirically determined effective diffusivity which takes care of the structure of solid. A typical problem of liquid transfer in a solid, of interest, is drying of solids.
Related Topics