Chapter: 11th Biochemistry : Chapter 7 : Nucleic Acids
Composition of Nucleic acids
Composition
Nucleic
acids are very long, thread-like polymers, made up of a linear array of
monomers called nucleotides held together by phosphodiester bridges.
Nucleotides have three characteristic components (i) Base (ii) Sugar (iii)
Phosphate group.
1. Common Bases of Nucleic acids
The
bases of nucleic acids are heterocyclic, containing aromatic ring in their
structures. They may be monocyclic pyrimidines or bicyclic purines.
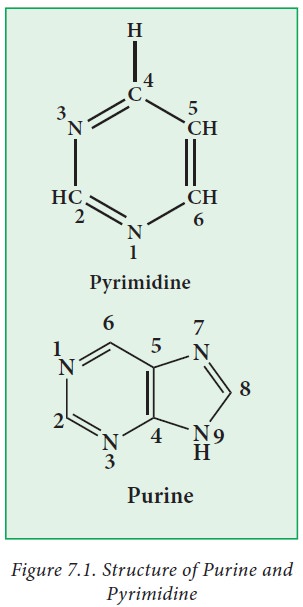
i. Pyrimidine bases
Pyrimidinesare
six-membered heterocyclic aromatic rings containing two nitrogen atoms (Figure
7.1) Pyrimidine ring is numbered in the clock-wise fashion. The common
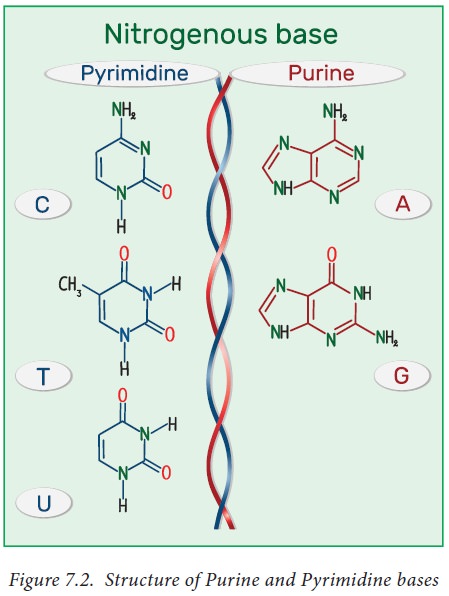
naturally occurring pyrimidines are cytosine, uracil, and thymine
(5-methyluracil) (Figure 7.2). Cytosine and thymine are the pyrimidines
typically found in DNA, whereas cytosine and uracil are common in RNA.
Properties of Pyrimidine bases
· Pyrimidines are basic in nature. They are less soluble in water.
· They absorb UV light at 260 nm. This property is
used to detect and estimate DNA and RNA in biological solutions.
· They are capable of forming hydrogen bonds with
purine nucleotides in nucleic acids.
· They exhibit keto–enol tautomerism.
ii. Purine bases
Purine
is a bicyclic ring formed by fusion of pyrimidine ring with imidazole ring.
Purine ring is numbered in the anti-clockwise fashion. Adenine (6-amino purine)
and guanine (2-amino-6-oxy purine), the two common purines, are found in both
DNA and RNA (Figure 7.2 ). Other naturally occurring purine derivatives include
hypoxanthine, xanthine, and uric acid. Hypoxanthine and xanthine are found only
rarely as constituents of RNA, while they are intermediates (compounds formed
in the synthetic pathway) in the synthesis of nucleic acids Uric acid is the
catabolic end product of nucleic acids.
Properties of Purine bases
·
Purines are basic in nature. They are sparingly soluble in water.
·
They also absorb UV light at 260 nm. This property is used to detect and
estimate DNA and RNA in biological solutions.
·
They are capable of forming hydrogen bonds with pyrimidine nucleotides
in nucleic acids.
·
They exhibit keto –enol tautomerism.
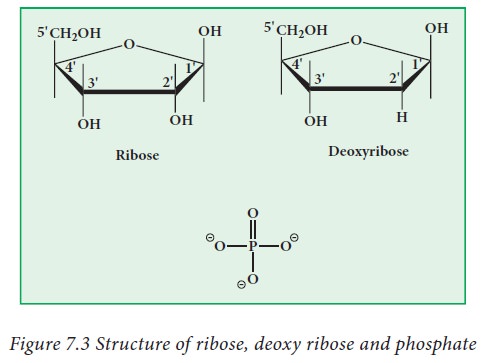
2. Sugars
There are two types of sugars present
in nucleic acids. They are ribose and deoxyribose (Fig.7.3). Based on the sugar
moiety present in the nucleic acids, they are classified as Deoxy ribonucleic
acid (DNA) and Ribonucleic acid (RNA). DNA is present in the nucleus,
mitochondria and chloroplasts. RNA is present in the nucleus, nucleolus,
ribosome and cytoplasm. Ribose and deoxy ribose have differences in their
properties. Deoxy ribose is less reactive in nature, when compared to ribose.
3. Phosphate
Phosphoric acid forms phospho-diester
linkage between nucleosides. Based on the number of phosphate group present in
the nucleotide, they are classified as monophosphates, diphosphates and
triphosphates.
Related Topics