Science - Colloidal Solutions | 9th Science : Matter Around Us
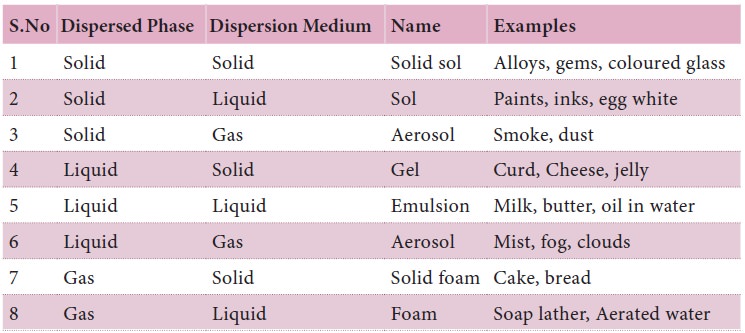
Chapter: 9th Science : Matter Around Us
Colloidal Solutions
Colloidal Solutions
A colloidal solution is a heterogeneous system
consisting of the dispersed phase and the dispersion medium.
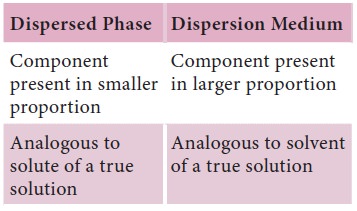
Classification of colloids based on physical state of dispersed phase and dispersion medium
Dispersed phase or the dispersion medium can be a
solid, or liquid or gas. There are eight different combinations possible (The
combination in which both the dispersed phase and dispersion medium are gases
which are completely miscible and can never give rise to a colloidal solution).
Because gas in gas formed a true solution.
We can see that the particle size in a colloidal
solution is in between that of a true solution and suspension. Because of this particular
range in size colloidal solutions show certain special properties like Brownian
movement and Tyndall effect. You are already familiar with the Brownian
movement and the particle nature of matter is explained on that.
1. Brownian movement
It is a kinetic property. When colloidal solution
are viewed under powerful microscope, it can be seen that colloidal particles
are moving constantly and rapidly in zig-zag directions. The Brownian movement
of particles is due to the unbalanced bombardment of the particles by the
molecules of dispersion medium.
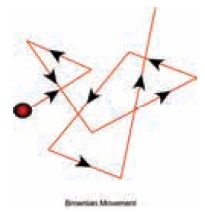
2. Tyndall effect

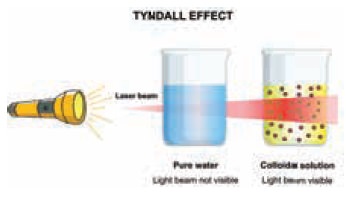
Cause for Tyndall effect
This phenomenon is due to scattering of light by colloidal particles. The colloidal particles become self-luminous due to absorption of light energy which is then scattered from their surface. The maximum scattered intensity in the plane is at right angle to the path of the light and thus the path becomes visible when observed from the sides. The intensity of scattered light depends on the type of colloidal solution and the size of the colloidal particles.
Think and answer
1. Why whole
milk is white?
2. Why ocean
is blue?
3. Why sun
looks yellow when it is really not?
Some Important Types of Colloids
3. Gels
Gels are colloidal solutions with liquid dispersed
in solid. A gel is a semi-solid substance which can ow but not as freely as a
liquid. Within a gel the solid (dispersion medium) makes a kind of network
which traps the dispersed liquid and makes it unable to ow freely.
Hair creams that are used to keep hair in place are
gels that contain water and an oil.

Foam and Solid foams: when gas dispersed in a
liquid is called a foam. E.g. soap bubbles, carbonated beverages etc.
When the gases are dispersed in a solid structure
is called solid foam. E.g. Bread, mattresses.

Emulsions - a special kind of colloids
An emulsion is a colloid of two or more immiscible
liquids where one liquid is dispersed in another liquid. This means one type of
liquid particles get scattered in another liquid. In other words, an emulsion
is a special type of mixture made by combining two liquids that normally don’t
mix. The word emulsion comes from the Latin word meaning “to milk” (milk is one
example of an emulsion of fat and water). The process of turning a liquid
mixture into an emulsion is called emulsification.
Examples of emulsions
Milk, butter, cream, egg yolk, paints, cough
syrups, facial creams, pesticides etc. are some common examples of emulsions.
Types of emulsions
The two liquids mixed can form different types of
emulsions. For example, oil and water can form an oil in water emulsion, where
the oil droplets are dispersed in water, or they can form a water in oil
emulsion, with water dispersed in oil.

Emulsions nd wide applications in food processing,
pharmaceuticals, metallurgy and many other important industries.

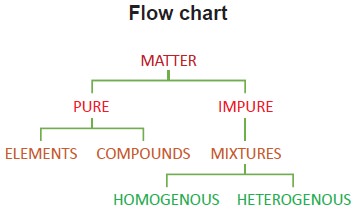
Classification of matter based on composition – summary
Related Topics