Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Insulin
Clinical and Practice Aspects of Insulin
CLINICAL AND PRACTICE ASPECTS
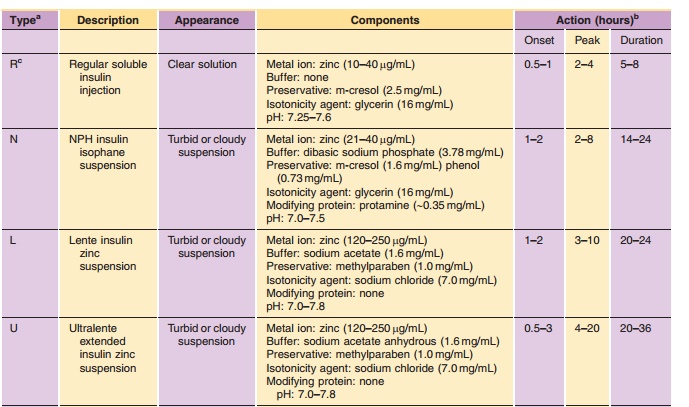
Vial Presentations
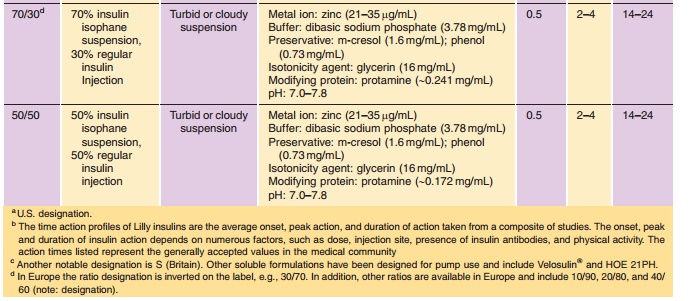
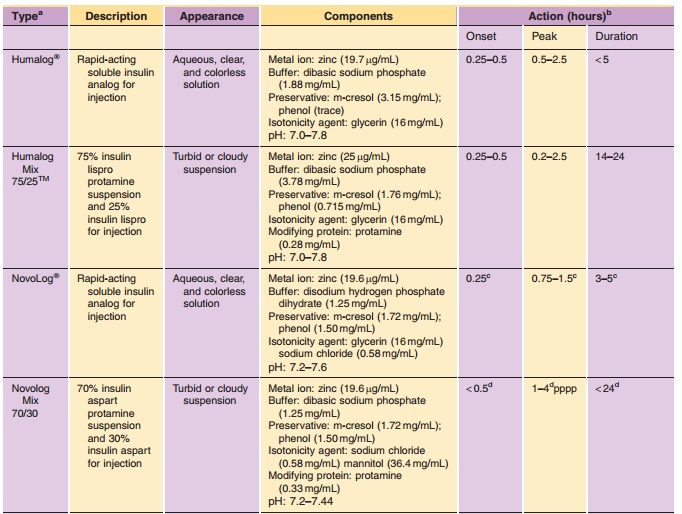
Insulin is commonly available in 10-mL vials. In the United States, a strength of U-100 (100 U/mL) is the standard, whereas outside the United States both U-100 and U-40 (40 U/mL) are commonly used. It is essential to obtain the proper strength and formula-tion of insulin in order to maintain glycemic control. In addition, species and brand/method of manufac-ture are important. Any change in insulin should be made cautiously and only under medical supervision (Galloway, 1988; Brackenridge, 1994). Common for-mulations, such as Regular, NPH, and Lente, are listed in Table 2 and the newer insulin analog formulations are listed in Table 3. Mixtures of rapid-or fast-acting with intermediate-acting insulin for-mulation, e.g., NPH/Regular 70/30, NPH/Regular 50/50, Humalog Mix 75/25, and NovoLog Mix 70/30, are a popular choice for glycemic control. The ratio is defined as ratio of protamine-containing fraction/ rapid-or fast-acting fraction, e.g., Humalog Mix 75/25 where 75% of a dose is available as insulin lispro protamine suspension and 25% insulin lispro for injection. With regards to NPH:Regular mixtures, caution must be used in the nomenclature because it may vary depending on the country of sale and the governing pharmacopeial body. In the United States, for example, the predominant species is listed first as in N/R 70/30, but in Europe the same formulation is described as R/N 30/70 (Soluble/Isophane) where the base (“normal”) ingredient is listed first. Currently an effort is being made to standardize worldwide to the European nomenclature. Mixtures available in the United States include N/R 70/30 and 50/50 while Europe has R/N 10/90, 20/80, 30/70, 40/60, and 50/50.
Injectors
Insulin syringes should be purchased to match the strength of the insulin that is to be administered (e.g., for U-100 strength use 30- or 100-unit syringes designated for U-100). The gauge of needles available for insulin administration has been reduced to very fine gauges (30–31 gauges) in order to minimize pain during injection. The use of a new needle for each dose maintains the sharp point of the needle and ensures a sterile needle for the injection.
In recent years the availability of insulin pen injectors has made dosing and compliance easier for the patient with diabetes. The first pen injector used a 1.5-mL cartridge of U-100 insulin. A needle wasattached to the end of the pen, and the proper dose was selected and then injected by the patient. The cartridge was replaced when the contents were exhausted, typically 3 to 7 days. Currently, 3.0-mL cartridges in U-100 strength for Regular, NPH, and the range of N/R mixtures have become the market standard with regard to size and strength. The advantages of the pen injectors are primarily better compliance for the patient through a variety of factors including more accurate and reproducible dose control, easier transport of the drug, more discrete dose administration, timelier dose administration, and greater convenience.
Continuous Subcutaneous Insulin Infusion - External Pumps
As previously mentioned, solution formulations of human insulin specifically designed for continuous subcutaneous insulin infusion (CSII) are commercially available. CSII systems were traditionally used by a small population of diabetic patients, but have become more popular with the recent introduction of rapid-acting insulin analogs. Currently, all three rapid-acting insulin analog formulations have re-ceived regulatory approval for this mode of delivery; however, specific in vitro data demonstrating the appropriate physicochemical stability for CSII has only been reported for insulin lispro (DeFelippis et al., 2006). Pump devices contain glass or plastic reservoirs that must be hand-filled from vial presentations by the patient. Some pumps have been specifically designed to accept the same glass 3-mL cartridges used in pen injector systems. Due to concerns over the impact of elevated temperature exposure and me-chanical stress on the integrity of the insulin molecule along with the increased risk of microbial contamina-tion, reservoirs, infusion sets, catheters and insulin must be replaced and a new infusion site selected every 48 hr or less.
Noninvasive Delivery
Since the discovery of insulin there has been a strong desire to overcome the need for injection-based therapy. Progress has been made in the form of needle-free injector systems (Robertson et al., 2000), but these devices have not gained widespread acceptance presumably because administration is not entirely pain free, device costs are high and other factors make it less desirable than traditional injection. Extensive research efforts have also focused on noninvasive routes of administration with attempts made to demonstrate the feasibility of transdermal, nasal, buccal, ocular, pulmonary, oral and even rectal delivery of insulin. Unfortunately, most attempts failed to progress beyond the proof of concept stage because low bioavailability, dose response variabilityand other adverse factors seriously called into ques-tion commercial viability. This situation has changed for pulmonary and buccal delivery of insulin as products for these routes of administration have been recently approved by regulatory authorities.
Several pulmonary delivery systems specifically aimed at insulin administration have advanced suffi-ciently through development to enable more extensive studies in human clinical trials and comprehensive reviews examining this work in detail are available (Patton et al., 1999, 2004; Cefalu, 2004). While many of these technologies continue to advance through late-stage development, one insulin pulmonary delivery system, referred to as Exubera , has recently received regulatory approval in both Europe and the United States. Exubera consists of a dry powder insulin formulation composed of small geometric diameter particles produced by spray drying (Patton, 1998). The powder formulation is packaged into individual blisters and combined with an active device that incorporates a mechanical energy source to achieve dispersion and aerosolization of the particles.
Based on the published clinical trial informa-tion, some generalizations about pulmonary insulin administration can be made (Heinemann et al., 2001; Patton et al., 2004). The pharmacokinetic profile of inhaled insulin is characterized by a faster onset compared to subcutaneous administration of solu-tion insulin formulations and at least as fast as rapid-acting insulin analogs; thus, the current delivery systems and formulations are targeted at controlling postprandial glucose excursions. Duration of action is intermediate between that of subcutaneously administered rapid-acting insulin analogs and reg-ular insulin. While the pharmacological properties reported for inhaled insulin seems appropriate to meet prandial insulin requirements, it will not address basal insulin needs. In certain treatment regimes, an injection of a long-acting (basal) insulin preparation will still be required to adequately manage blood glucose levels. Intrapatient variability in pharmacokinetic and pharmacodynamic re-sponses for pulmonary-delivered insulin is low and similar to subcutaneously administered insulin. Estimated bioavailabilities are typically in the range of approximately 10–20% relative to insulin adminis-tered by subcutaneous injection. In special patient populations such as smokers, absorption of insulin is significantly greater (Himmelmann et al., 2003) when administered by the pulmonary route. Alterations in pharmacological responses have been similarly ob-served in other studies conducted in elderly subjects, asthmatics, or individuals with acute respiratory tract infections (Patton et al., 2004). Now that a pulmonary insulin product is commercially avail-able, knowledge about this noninvasive route ofadministration will continue to increase as broader patient experience is obtained.
In addition to pulmonary insulin, a buccal insulin product, referred to as OralinTM, has been developed consisting of a solution formulation of insulin containing various absorption enhancers needed to achieve mucosal absorption (Modi et al., 2002) and a metered dose inhaler is used to administer a fine mist into the oral cavity. Clinical study results evaluating this buccal delivery system in healthy subjects as well as patients with Type 1 and Type 2 diabetes have been reported (Modi et al., 2002; Cernea et al., 2004). While Oralin has only achieved regula-tory approval in Ecuador, it is unknown if the product will have similar success with regulatory agencies in the United States or Europe.
Storage
Insulin formulations should be stored in a cool place that avoids direct sunlight. Vials or cartridges that are not in active use should be stored under refrigerated (2–8LC) conditions. Vials or cartridges in active use may be stored at ambient temperature. High tem-peratures, such as those found in non-air-conditioned vehicles in the summer, should be avoided. Insulin formulations should not be frozen; if this occurs, the product should be disposed immediately. Insulin formulations should never be purchased or used past the expiration date on the package.
Usage
Resuspension
Insulin suspensions (e.g., NPH, Premixtures, Lente, Ultralente) should be resuspended by gentle back-and-forth mixing and rolling of the vial between the palms to obtain a uniform, milky suspension. The patient should be advised of the resuspension technique for specific insoluble insulin and insulin analog formulations, which is detailed in the package insert. The homogeneity of suspensions is critical to obtaining an accurate dose. Any suspension that fails to provide a homogeneous dispersion of particles should not be used. Cartridges in pen injectors may be suspended in the same manner. However, the smaller size of the container and shape of the injector device may require slight modification of the resuspension method. A glass bead is typically added to cartridges to aid in the resuspension of suspension formulations.
Dosing
Dose withdrawal should immediately follow the resus-pension of any insulin suspension, especially Lente and Ultralente formulations because they settle relatively quickly. The patient should be instructed by his or her doctor, pharmacist, or nurse educator in properprocedures for dose administration. Of particular importance are procedures for disinfecting the container top and injection site. The patient is also advised to use a new needle and syringe for each injection. Reuse of these components, even after cleaning, may lead to contam-ination of the insulin formulation by microorganisms or by other materials, such as cleaning agents.
Extemporaneous Mixing
As discussed above in the section on “Intermediate-and Long-acting Insulin Preparations,” Regular in-sulin can be mixed in the syringe with NPH, Lente, and Ultralente. However, only the Regular/NPH mixtures are stable enough to be stored for extended periods of time. The Lente/Regular and Ultralente/ Regular formulations can be prepared but must be used immediately. Otherwise, the time-action of the Regular component can be affected.
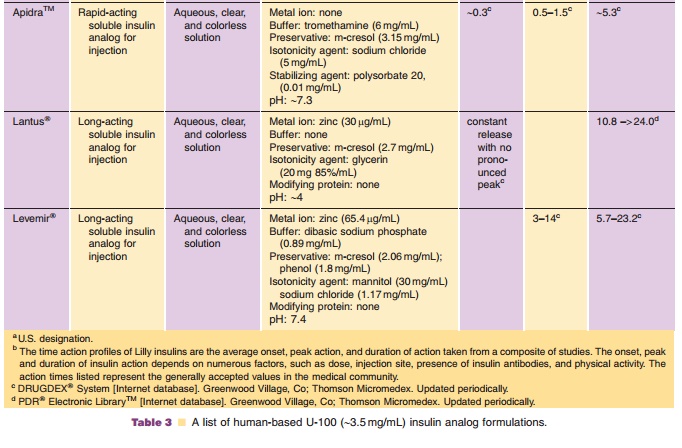
With regards to extemporaneously mixing of the newer insulin analogs, caution must be used. Lantus, due to its acidic pH, should not be mixed with other fast- or rapid-acting insulin formulations which are formulated under neutral pH. If Lantus is mixed with other insulin formulations, the solution may become cloudy due to pI precipitation of both the insulin glargine and the fast- or rapid-acting insulin resulting from pH changes. Consequently, the pharmacoki-netic/pharmacodynamic profile, e.g., onset of action, time to peak effect, of Lantus and/or the mixed insulin may be altered in an unpredictable manner. With regard to rapid-acting insulin analogs, extem-poraneous mixing with human insulin NPH formula-tions is acceptable if used immediately. Not under any circumstances should these formulations be stored, as human insulin and insulin analog exchange can occur between solution and the crystalline matter thereby potentially altering time-action profiles of the solution insulin analog. With regard to Levemir, mixing restrictions have not been reported.
Related Topics