Chapter: Biology of Disease: Cancer
Clinical Tests for Cancer - General Diagnosis of Cancer
GENERAL DIAGNOSIS OF CANCER
The symptoms of cancer at presentation depend on the location and extent of the tumor. Depending on the cancer, symptoms may develop when the cancer is relatively small as, for example, when even a small tumor of the brain causes pressure to develop. Other tumors, such as those developing in the ovary may not produce symptoms until it is relatively large.
A number of general signs may alert an individual to the presence of an undiagnosed tumor, for example, an unexplained weight loss of about 5 kg or more. Weight loss that occurs as a result of cancers is due to the release of cytokines from cells of the immune system. In end-stage cancer, this weight loss is known as cachexia. Fever, which is also induced by cytokines, is often found in patients with advanced cancer, although this may also be an early warning of certain types of cancer. An example of this is Hodgkin’s lymphoma, which is characterized by fever, often during sleep, and is accompanied by drenching night sweats. Fatigue may also be a symptom, particularly where the cancer causes a loss of blood with concomitant anemia, as may occur, for example, with stomach cancer. Other signs and symptoms include the presence of a lump, as for example in the breast or testicles, or unusual bleeding or discharges.
CLINICAL TESTS FOR CANCER
Clinical tests for cancer are used to screen for cancer in an ‘at-risk’ population, to detect cancer in a patient presenting indicative symptoms, to monitor the success of treatment or to detect recurrence in a patient who has been in remission. These tests fall into a number of categories. They may involve the detection and/or quantification of tumor associated molecules, the detection and localization of tumors within the body, and the histological examination of biopsies from suspect tissue to determine the nature of the tumor and/or to detect precancerous conditions.
Detection and estimation of tumor-associated molecules
Some types of tumor are associated with increases in tumor associated molecules in the blood. These ‘tumor associated antigens’ are detected and quantified by using monoclonal antibodies specific for the antigen in question. The antibody may be used in an enzyme-linked immunosorbent assay (ELISA) or in radioimmunoassay (RIA) as described. Examples of tumor associated antigens include prostate-specific antigen which is elevated in the blood of patients with cancer of the prostate, and CA 125 antigen, which is found in the blood of women with ovarian cancer. Carcinoembryonic antigen (CEA) is a glycoprotein that is overproduced in most colon carcinomas and in carcinoma of the lung and breast. Serum concentrations are measured and used to monitor treatment and to predict prognosis.
Unfortunately, increases in tumor associated antigens are not exclusive to cancer so that the tests can only give an indication of cancer. Increased
Detection and localization of tumors within the body
Patients who present with symptoms indicative of cancer may be examined using diagnostic imaging procedures in order to localize the potential tumor or to determine the extent of metastasis. Diagnostic imaging may involve the use of X-rays, computed tomography (CT) also known as computed axial tomography (CAT), magnetic resonance imaging (MRI), positron emission tomography (PET) and ultrasound, all of which are undertaken by clinical radiologists.
The routine screening for breast cancer by mammography is offered to women in the UK over the age of 50 years. A tumor will show up as a shadow on a breast mammogram. Patients who have lung cancer may have X-rays taken of their bones, in order to determine whether the tumor has spread to them.
Computed axial tomography scanners were introduced into hospitals in the UK in 1975. The technique uses X-rays to take sequential pictures of the body from different directions. In practice, the patient may be required to drink a contrast solution, or this may be administered intravenously, to enhance the tissue contrast. The patient then lies still on a table that passes through the X-ray machine, which rotates around the patient taking pictures of thin ‘slices’ of tissue. Computers then combine the images to produce three-dimensional images or computed tomograms. Magnetic resonance imaging (MRI) and positron emission tomography (PET), outlined, may also be used to visualize abnormal growths within the body and for determining the extent of tumor growth. Ultrasound may be used to locate tumors within the abdomen, including tumors of the liver and ovaries. However, in order to determine whether the abnormal growth represents a cancer or a benign lesion, it is essential to examine biopsies of the growth.
Biopsies and histology
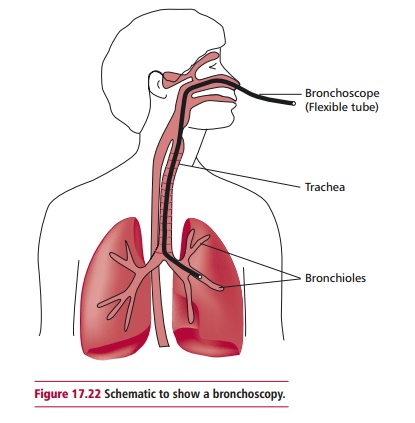
Biopsy material may be obtained from a variety of sources in one of several ways. Samples from solid tumors may be obtained by endoscopy, or during surgical procedures involving local or general anesthesia. An endoscope is a long thin flexible tube with a camera and light on the end. Depending on the tumor, the endoscope is inserted into a body cavity and allows internal tissues such as the GIT and the lungs to be viewed. Endoscopes also enable samples of the suspect tissue to be removed without the need for surgery. One example of the use of endoscopy is in bronchoscopy, which is used to obtain biopsies of lung tissue in suspected cases of lung cancer.
Needle biopsy allows small amounts of tissue to be obtained from a variety of solid tissues. Samples of blood and bone marrow may be examined to detect leukemias. Some solid tumors may cause the build up of fluid containing cancer cells within the peritoneal cavity , where it is known as ascites fluid, or in the thorax, where a pleural effusion may develop . These fluids contain tumor cells in suspension and may be examined histologically to confirm the presence of cancer cells. Finally, some precancerous lesions may be detected by taking smears of tissue and examining them histologically, as for example in the preparation of cervical smears in order to detect precancerous lesions as outlined below.
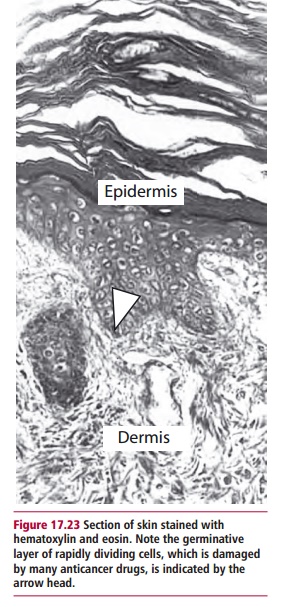
Solid tissue obtained for histological examination must be processed in order to obtain thin sections for microscopic examination. The material is first preserved by fixation in, for example, formalin, and then dehydrated and embedded in paraffin wax to support the tissue. This allows sections between 2 and 7 Mm thick to be cut using a microtome. The sections are then mounted on slides, dewaxed with xylene, rehydrated and then stained in the manner appropriate to the investigation. The commonest stains for tissue sections are hematoxylin and eosin. Hematoxylin stains the nucleus purple/ black depending on the formulation, while the eosin stains the cytoplasmic contents pink. Hematoxylin and eosin allow good differentiation within the tissue (Figure 17.23). Smears of blood and bone marrow are most frequently stained with May-Grunwald-Giemsa, which stains the nuclei blue, while the cytoplasmic contents stain differentially depending on the cell type. This stain therefore allows differentiation between different types of leukocytes. The stain commonly used to detect precancerous cells in cervical smears is the Papanicolaou stain, or Pap stain as it is more frequently known. This formulation contains five different stains: hematoxylin, which stains the nucleus, Orange G (OG-6) and EA, which contains light green, eosin Y and Bismarck brown Y. Orange G and EA are counterstains. The Pap stain is often used to stain buccal and sputum smears as well as those obtained from the cervix.
The preparation of material from solid tissues for histological examination is a long process. Sometimes a more rapid examination may be required as, for example, when the result of a biopsy is needed during an operation in order to determine the extent of surgery required. In such cases, the biopsy is rapidly frozen to –176°C by immersion in liquid nitrogen. This process hardens the material and allows it to be cut into 5–10 Mm sections using a freezing microtome or cryostat. Once the sections have been prepared they can be stained with hematoxylin and eosin without the long procedures required with paraffin sections, often within minutes of removal from the body. Examination will then, hopefully, ensure the appropriate surgical procedure.
It is possible to stain cancer cells more specifically if they bear a tumor-specific marker, such as CEA (see above). Cryostat sections are often used for this process, because fixation, wax embedding and clearing can destroy some antigenic sites on the tumor. The sections are stained by immunohistochemistry . In this process, the sections are incubated with a monoclonal antibody to a tumor associated antigen. This specific binding is visualized by incubation with an enzyme-labeled anti-immunoglobulin, followed by a further incubation with the appropriate substrate. For enzyme immunohistochemistry, a colorless substrate is chosen that produces a colored insoluble product. It is now possible to use paraffin embedded sections for immunohistochemistry because the antigenic sites that were destroyed during the preparation process, can be ‘retrieved’ by microwaving the sections in water, subjecting them to heat at pressure using a pressure cooker or by allowing the sections to be partially digested with a proteolytic enzyme. The length of time required to retrieve the antigenic sites has to be determined by trial and error, using positive and negative control slides.
Molecular diagnosis
The histological diagnosis of potentially cancerous cells is increasingly being supported by molecular diagnostic techniques and it is probable that molecular methods will be used more frequently across a greater range of tumors once the genetic basis for the development of these tumors has been established. The determination of the genetic profile of a patient’s primary tumor will no doubt become routine and this will inform treatment and be a predictor of prognosis. One way in which tumors have been investigated
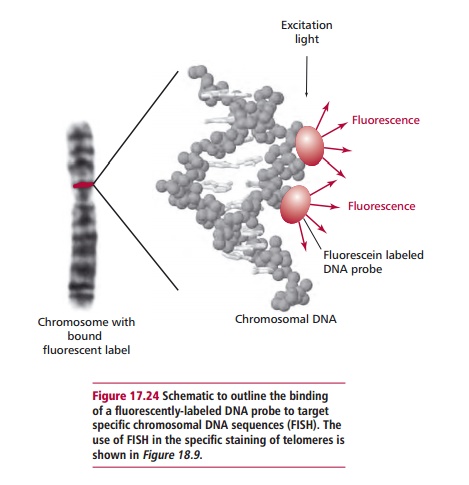
is to extract DNA using a fine needle biopsy, to amplify the DNA by using the polymerase chain reaction (PCR;), and to analyze the DNA obtained for mutations known to be implicated in cancer. Fluorescence in situ hybridization technique (FISH;) is also applied to diagnosis. Thin sections of the tumor are treated to separate the DNA strands which are then hybridized in situ with fluorochrome labeled probes for relevant mutated, cancer associated genes (Figure 17.24). The slides are then examined using a fluorescence microscope. The presence of the mutation is indicated by fluorescent spots in the nucleus.
Related Topics