Chapter: 11th 12th std standard Class Physics sciense Higher secondary school College Notes
Classification of nuclei and Properties of nucleus Nuclear size
The atomic nucleus was discovered by Earnest
Rutherford in 1911. Rutherford's experiment on scattering of alpha particles
proved that the mass of the atom and the positive charge is concentrated in a
very small central core called nucleus. The dimension of nucleus is much
smaller than the overall dimension of the atom. The nucleus is surrounded by
orbiting electrons.
Nucleus
The nucleus consists of the elementary
particles, protons and neutrons which are known as nucleons. A proton has
positive charge of the same magnitude as that of electron and its rest mass is
about 1836 times the mass of an electron. A neutron is electrically neutral,
whose mass is almost equal to the mass of the proton. The nucleons inside the
nucleus are held together by strong attractive forces called nuclear forces.
A nucleus of an element is represented as ZXA,
where X is the chemical symbol of the element. Z represents the atomic number
which is equal to the number of protons and A, the mass number which is equal
to the total number of protons and neutrons. The number of neutrons is
represented as N which is equal to A−Z. For example, the chlorine nucleus is represented as 17Cl35. It contains 17 protons
and 18 neutrons.
1Classification of nuclei
(i) Isotopes
Isotopes are atoms of the same element having
the same atomic number Z but different mass number A. The nuclei 1H 1,
1H2 and 1H3 are the isotopes of
hydrogen. In other words isotopes of an element contain the same number of
protons but different number of neutrons. As the atoms of isotopes have
identical electronic structure, they have identical chemical properties and
placed in the same location in the periodic table.
(ii) Isobars
Isobars are atoms of different elements having
the same mass number A, but different atomic number Z. The nuclei 8O16
and 7N16 represent two isobars. Since isobars are atoms
of different elements, they have different physical and chemical properties.
(iii) Isotones
Isotones are atoms of different elements having
the same number of neutrons. 6C14 and 8O16
are some examples of isotones.
2.General properties of nucleus Nuclear size
According to Rutherford's á−particle scattering experiment, the distance of the closest
approach of á − particle to the nucleus was taken as a measure
of nuclear radius, which is approximately 10−15m. If the nucleus is assumed to be spherical,
an empirical relation is found to hold good between the radius of the nucleus R
and its mass number A. It is given by
R ∝∝ A1/3
R = roA1/3
where ro is the constant of
proportionality and is equal to 1.3 F (1 Fermi, F = 10−15 m)
Nuclear density
The nuclear density ñN can be calculated from the mass and size of
the nucleus.
ρN = Nuclear mass / Nuclear volume
Nuclear mass = AmN
where, A = mass number
and mN = mass of one nucleon and is
approximately equal to 1.67 × 10−27 kg
Nuclear volume = 4/3 πR3
ρN = mN / ( 4/3 πr03)
Substituting the known values, the nuclear
density is calculated as 1.816 × 1017 kg m−3 which is almost a constant for all the nuclei
irrespective of its size.
The high value of the nuclear density shows
that the nuclear matter is in an extremely compressed state.
Nuclear charge
The charge of a nucleus is due to the protons
present in it. Each proton has a positive charge equal to 1.6 × 10−19 C.
The
nuclear charge = Ze, where Z is the atomic number.
Atomic mass unit
It is convenient to express the mass of a
nucleus in atomic mass unit (amu), though the unit of mass is kg. One atomic
mass unit is considered as one twelfth of the mass of carbon atom 6C
12. Carbon of atomic number 6 and mass number 12 has mass equal to
12 amu.
1 amu = 1.66 × 10−27 kg
The mass of a proton, mp = 1.007276
amu
This is equal to the difference in mass of the
hydrogen atom which is 1.007825 amu and the mass of electron.
The mass of a neutron, mn = 1.008665
amu
The energy equivalence of one amu can be
calculated in electron-volt
Einstein's mass energy relation is, E = mc2 Here, m = 1
amu = 1.66 × 10−27 kg
c = 3 × 108 ms−1
E = 1.66
× 10−27 × (3 ×
108)2 J
One electron-volt (eV) is defined as the energy
of an electron when it is accelerated through a potential difference of 1 volt.
1 eV = 1.6 × 10−19 coulomb × 1 volt 1 eV = 1.6 × 10−19 joule
Hence, E = 1.66
× 10 − 27 × (3 ×10 8 )2
/ 1.6 ×10-19 eV
= = 931 million electronvolt = 931 MeV
Thus, energy equivalent of 1 amu = 931 MeV
Nuclear mass
As the nucleus contains protons and neutrons,
the mass of the nucleus is assumed to be the mass of its constituents.
Assumed nuclear mass = ZmP + Nmn,
where mp and mn are the mass of a proton and
a neutron respectively. However, from the measurement of mass by mass
spectrometers, it is found that the mass of a stable nucleus (m) is less than
the total mass of the nucleons.
i.e mass
of a nucleus, m < (Zmp + Nmn) Zmp + NmN
- m = ∆m
where ∆m is the mass defect
Thus, the difference in the total mass of the
nucleons and the actual mass of the nucleus is known as the mass defect.
Note : In any mass spectrometer, it is possible
to determine only the mass of the atom, which includes the mass of Z electrons.
If M represents the mass of the atom, then the mass defect can be written as
∆m = ZmP + Nmn + Zme
- M
= ZmH + Nmn - M
where mH represents the mass of one
hydrogen atom
Binding energy
When the protons and neutrons combine to form a
nucleus, the mass that disappears (mass defect, ∆m) is converted into an equivalent amount of energy (∆mc2). This energy is called the binding energy of the
nucleus.
Binding energy = [ZmP
+ Nmn - m] c2
= ∆m c2
The binding energy of a nucleus determines its
stability against disintegration. In other words, if the binding energy is
large, the nucleus is stable and vice versa.
The binding energy per nucleon is
BE/ A = Binding energy of the nucleus
/ Total number of nucleons
It is found that the binding energy per nucleon
varies from element to element. A graph is plotted with the mass number A of
the nucleus along the X−axis and
binding energy per nucleon along the Y-axis (Fig).
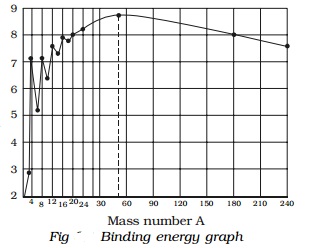
Explanation of binding energy curve
i.
The binding energy per nucleon increases
sharply with mass number A upto 20. It increases slowly after A = 20. For
A<20, there exists recurrence of peaks corresponding to those nuclei, whose
mass numbers are multiples of four and they contain not only equal but also
even number of protons and neutrons. Example: 2He4, 4Be8,
6C12, 8O16, and 10Ne20.
The curve becomes almost flat for mass number between 40 and 120. Beyond 120,
it decreases slowly as A increases.
ii.
The
binding energy per
nucleon reaches a
maximum of MeV at A=56,
corresponding to the iron nucleus (26Fe56). Hence, iron
nucleus is the most stable.
iii.
The average binding energy per nucleon is about
8.5 MeV for nuclei having mass number ranging between 40 and 120. These elements
are comparatively more stable and non radioactive.
iv.
For higher mass numbers the curve drops slowly
and the BE/A is about 7.6 MeV for uranium. Hence, they are unstable and
radioactive.
v.
The lesser amount of binding energy for lighter
and heavier nuclei explains nuclear fusion and fission respectively. A large
amount of energy will be liberated if lighter nuclei are fused to form heavier
one (fusion) or if heavier nuclei are split into lighter ones (fission).
Related Topics