Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Chromatography and Different Chromatographic Techniques
Chromatography
This technique was introduced by M.S.Tswett, a Russian botanist in 1906
when he reported the separation of different coloured constituents of
chlorophyll. He achieved it by passing a petroleum ether solution of the
chlorophyll present in leaves through a column of calcium carbonate firmly
packed into a narrow glass tube. Different components of the pigment got
separated into band or zones of different colours.
Chromatography is based on the general principle of distributing the
components of a mixture of organic compounds between two phases - a stationary
phase and a moving phase. The stationary phase can be a solid or liquid
supported on a solid, while the moving phase is a liquid or a gas. When the
stationary phase is a solid, the basis of separation is adsorption; when it is
a liquid, the basis is partition.
Hence, chromatography can be defined as the
technique for the separation of a mixture of compounds where the separation is
brought about by the differential movement of the individual compounds through
a porous medium under the influence of a moving solvent. The technique has now
a days undergone tremendous modification and is widely used for the separation
and purification of different types of organic compounds.
The different chromatographic techniques used are : column
chromatography (CC), thin-layer chromatography (TLC), paper chromatography
(PC), gas-liquid chromatography (GLC) and ion-exchange chromatography.
a) Column
Chromatography
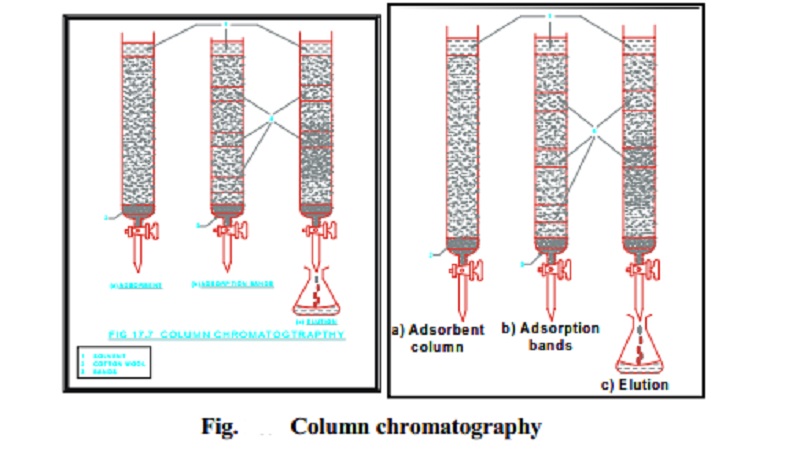
The simplest chromatographic method is column chromatography. It is
carried out in a long glass column having a stop-cock near the bottom. To start
the operation, a plug of cotton or glass wool is placed at the bottom of the
column to support the adsorbent powder. The tube is packed uniformly with
suitable adsorbent. This constitutes what is known as the stationary phase. The
commonly employed adsorbents are activated aluminium oxide (alumina), magnesium
oxide, silica gel and starch. A loose plug of cotton or glass wool is then
placed at the top of the adsorbent column.
The substance to be purified is added, as such if it is a liquid or in
the form of its solution in some suitable solvent if it is a solid, at the top
of the column and allowed to pass slowly through it. As it passes through the
column, the different components of a mixture (Say A, B and C) got adsorbed to
different extent and are thus retained by the adsorbent at different levels of
the column. The components which are adsorbed very strongly are retained at the
top while others are retained at lower levels. In this way different zones or
bands are formed in the column which contain different components of a mixture.
As soon as the last portion of the substances enter the column, a selected solvent,
known as eluent, is added to the column. This acts as moving phase. The
elements dissolve out the different components from the various zones
selectively and thus `take out' the different bands in the form of fractions
which are collected separately.
b) Thin Layer
Chromatography (TLC)
Thin layer chromatography (TLC) is another type of adsorption
chromatography, which involves separation of the substances of a mixture over a
thin layer of an adsorbent. A thin layer (about 0.2mm thick) of an adsorbent (Silica
gel or alumina) is spread over a glass plate of suitable size. The plate is
known as thin layer chromatography plate. The solution of the mixture to be
separated is applied as a small spot about 2 cm above one end of the TLC plate.
The glass plate is then placed in a closed jar containing the solvent (Below
2cm height). As the solvent jar moves up the plate, the components of mixture
move up along the plate to different distances depending on this degree of
adsorption and separation takes place. The relative adsorption of each
component of the mixture is expressed in terms of its retention factor ie., Rf
Value.
Rf = Distance
moved by the substance from base line (x) / Distance moved by the solvent from
base line (y) c)
c) Paper Chromatography
It is an important and useful class of partition chromatography. In this
technique, the stationary phase is considered to be made up of water molecules
bound to the cellulose network (inert support) of the paper. The mobile phase,
known as the developing solvent consists of either one solvent or a mixture of
different solvents. Separation of the mixture into pure compounds takes place
by the partitioning of different compounds
between
these two liquid phases. The mobile phase travels by capillary action through
the paper. Depending upon the way the solvent travels on the paper, there are
three types of paper chromatography.
1.
Ascending Paper Chromatography : The mobile
phase moves upwards on the paper strip in this case.
2.
Descending Paper Chromatography : The mobile
phase in this case moves downward on the paper strip.
3.
Circular or radial paper chromatography: The
mobile phase moves horizontally along a circular sheet of paper in this case.
In the ascending paper chromatography, the mixture of compounds is
applied on the paper as a spot little above the lower end and then this end is
dipped in the solvent. When the solvent has risen more than two third length of
the paper, then it is removed from the solvent. The paper is dried and is known
as chromatogram.
Now the spots for different compounds can be visualised using some
suitable chemicals. The ratio of the distance travelled by the compound in a
particular solvent to that the distance travelled by the solvent is a constant
and is known as retention factor (Rf). This value is used in identifying the
compounds.
Rf = Distance travelled by the compound / Distance travelled by the
solvent
Type of Chromatography Stationary Phase Mobile
Phase
1. Column Chromatography Solid Liquid
2. Thin Layer Solid Liquid
Chromatography (TLC)
3. Paper Chromatography Liquid Liquid
4. Gas Liquid Phase Liquid Gas
Chromatography
Difference
between paper chromatography and thin layer chromatography
Paper Chromatography
(i) Separation based on partition
(ii) Stationary
phase is the water molecules bound on the paper.
Thin Layer Chromatography
(i) Separation is based on partition, adsorption
and ion exchange.
(ii) Stationary
phase is a layer of silica gel or alumina on glass plate.
Related Topics