Example, Physical and Chemical properties - Chloroform - Trihaloalkane | 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Chloroform - Trihaloalkane
Trihaloalkane
Trihaloalkanes are compounds obtained by replacing three hydrogen atoms of a hydrocarbon by three halogen atoms.
Example

1) Chloroform
Chloroform is an important trihaloalkane. Dumas named CHCl3 as chloroform as it gives formic acid on hydrolysis.
Preparation:
Chloroform is prepared in the laboratory by the reaction between ethyl alcohol with bleaching powderfollowed by the distillation of the product chloroform. Bleaching powder act as a source of chlorine and calcium hydroxide. This reaction is called haloform reaction. The reaction proceeds in three steps as shown below.
Step – 1: Oxidation
CH3CH2OH + Cl2 → CH3CHO + 2HCl
Ethyl alcohol Acetaldehyde
Step – 2: Chlorination
CH3CHO + 3Cl2 → CCl3CHO + 3HCl
Acetaldehyde Trichloro acetaldehyde
Step – 3: Hydrolysis
2CCl3CHO + Ca(OH)2 → 2CHCl3 + (HCOO)2 Ca
Chloral   chloroform
Properties
Physical properties
i. Chloroform is a colourless liquid with peculiar sickly smell and a burning taste
ii. The vapours of chloroform when inhaled cause unconsciousness (depress the central nervous system) and hence it is used as an anaesthetic.
Chemical properties
1) Oxidation
Chloroform undergoes oxidation in the presence of light and air to form phosgene (carbonyl chloride)
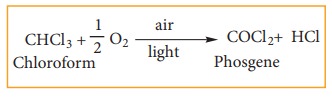
Since phosgene is very poisonous, its presence makes chloroform unfit for use as anaesthetic.
2) Reduction
Chloroform undergoes reduction with zinc and HCl in the presence of ethyl alcohol to form methylene chloride.

3) Nitration
Chloroform reacts with nitric acid to form chloropicrin.(Trichloro nitro methane)
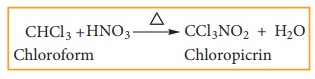
It used as an insecticide and soil sterilising agent.
4) Carbylamine reaction
Chloroform reacts with aliphatic or aromatic primary amine and alcoholic caustic potash, to give foul smelling alkyl isocyanide (carbylamines)
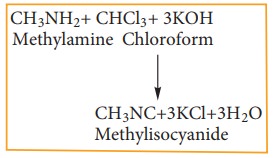
This reaction is used to test primary amine.
Related Topics