Chapter: Basic & Clinical Pharmacology : Pancreatic Hormones & Antidiabetic Drugs
Characteristics of Available Insulin Preparations
Characteristics of Available
Insulin Preparations
Commercial insulin
preparations differ in a number of ways, such as differences in the recombinant
DNA production techniques, amino acid sequence, concentration, solubility, and
the time of onset and duration of their biologic action.
A. Principal Types and Duration of Action of Insulin Preparations
Four principal types of injected insulins are available: (1) rapid-acting, with very fast onset and short duration; (2) short-acting, with rapid onset of action; (3) intermediate-acting; and (4) long-acting, with slow onset of action (Figure 41–5, Table 41–4). Injected rapid-acting and short-acting insulins are dispensed as clear solutions at neutral pH and contain small amounts of zinc to improve their stability and shelf life. Injected intermediate-acting NPH insulins have been modified to provide prolonged action and are dispensed as a turbid suspension at neutral pH with protamine in phosphate buffer (neutral protamine Hagedorn [NPH] insulin). Insulin glargine and insulin detemir are clear, soluble long-acting insulins.
The goal of
subcutaneous insulin therapy is to replicate normal physiologic insulin
secretion and replace the background or basal (overnight, fasting, and
between-meal) as well as bolus or prandial (mealtime) insulin. An exact
reproduction of the normal glycemic profile is not technically possible because
of the limitations inher-ent in subcutaneous administration of insulin. Current
regimens generally use insulin analogs because of their more predictable
action. Intensive therapy (“tight control”) attempts to restore near-normal
glucose patterns throughout the day while minimizing the risk of hypoglycemia.
Intensive regimens
involving multiple daily injections (MDI) use long-acting insulin analogs to
provide basal or background coverage, and rapid-acting insulin analogs to meet
the mealtime requirements. The latter insulins are given as supplemental doses
to correct transient hyperglycemia. The most sophisticated insulin regimen
delivers rapid-acting insulin analogs through a continu-ous subcutaneous
insulin infusion device. Conventional therapy consists of split-dose injections
of mixtures of rapid- or short-acting and intermediate-acting insulins.
1. Rapid-acting
insulin—Three
injected rapid-acting insulinanalogs—insulin
lispro, insulin aspart, and insulin
glulisine— are commercially available. The rapid-acting insulins permit
more physiologic prandial insulin replacement because their rapid onset
and
early peak action more closely mimic normal endogenous prandial insulin
secretion than does regular insulin, and they have the additional benefit of
allowing insulin to be taken immediately before the meal without sacrificing
glucose control. Their dura-tion of action is rarely more than 4–5 hours, which
decreases the risk of late postmeal hypoglycemia. The injected rapid-acting
insulins have the lowest variability of absorption (approximately 5%) of all
available commercial insulins (compared with 25% for regular insulin and 25% to
over 50% for long-acting analog for-mulations and intermediate insulin,
respectively). They are the preferred insulins for use in continuous subcutaneous
insulin infu-sion devices.
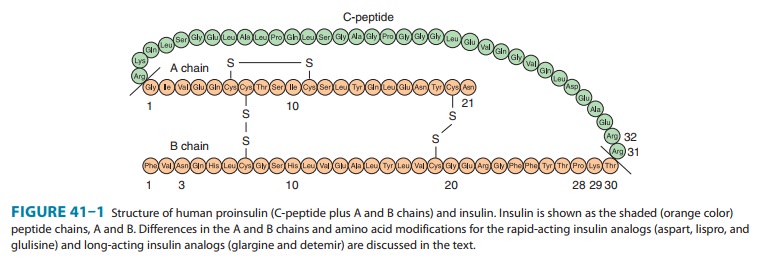
Insulin
lispro, the first monomeric insulin analog to be mar-keted, is produced by
recombinant technology wherein two amino acids near the carboxyl terminal of
the B chain have been reversed in position: Proline at position B28 has been
moved to B29, and lysine at position B29 has been moved to B28 (Figure 41–1).
Reversing these two amino acids does not interfere in any way with insulin
lispro’s binding to the insulin receptor, its circulating half-life, or its
immunogenicity, which are similar to those of human regular insulin. However,
the advantage of this analog is its very low propensity—in contrast to human
insulin—to self-associate in antiparallel fashion and form dimers. To enhance
the shelf life of insulin in vials, insulin lispro is stabilized into hexa-mers
by a cresol preservative. When injected subcutaneously, the drug quickly
dissociates into monomers and is rapidly absorbed with onset of action within
5–15 minutes and peak activity as early as 1 hour. The time to peak action is
relatively constant, regardless of the dose.
Insulin aspart is
created by the substitution of the B28 proline with a negatively charged
aspartic acid (Figure 41–1). This modifica-tion reduces the normal ProB28 and
GlyB23 monomer-monomer interaction, thereby inhibiting insulin
self-aggregation. Its absorption and activity profile are similar to those of
insulin lispro, and it is more reproducible than regular insulin, but it has
binding properties, activity, and mitogenicity characteristics similar to those
of regular insulin in addition to equivalent immunogenicity.
Insulin glulisine is
formulated by substituting a lysine for asparagine at B3 and glutamic acid for
lysine at B29. Its absorp-tion, action, and immunologic characteristics are
similar to those of other injected rapid-acting insulins. After high-dose
insulin glulisine interaction with the insulin receptor, there may be
down-stream differences in IRS-2 pathway activation relative to human insulin.
The clinical significance of such differences is unclear.
2. Short-acting insulin—Regular insulin is a
short-acting sol-uble crystalline zinc insulin that is now made by recombinant
DNA techniques to produce a molecule identical to that of human insulin. Its
effect appears within 30 minutes, peaks between 2 and 3 hours after
subcutaneous injection, and generally lasts 5–8 hours. In high concentrations,
eg, in the vial, regular insulin molecules self-aggregate in antiparallel
fashion to form dimers that stabilize around zinc ions to create insulin
hexamers. The hexameric nature of regular insulin causes a delayed onset and
prolongs the time to peak action. After subcutaneous injection, the insulin
hexamersare too large and bulky to be transported across the vascular
endothelium into the bloodstream. As the insulin depot is diluted by
interstitial fluid and the concentration begins to fall, the hex-amers break
down into dimers and finally monomers. This results in three rates of
absorption of the injected insulin, with the final monomeric phase having the
fastest uptake out of the injection site.
The clinical
consequence is that when regular insulin is admin-istered at mealtime, the
blood glucose rises faster than the insulin with resultant early postprandial
hyperglycemia and an increased risk of late postprandial hypoglycemia.
Therefore, regular insulin should be injected 30–45 or more minutes before the
meal to minimize the mismatching. As with all older insulin formulations, the
duration of action as well as the time of onset and the intensity of peak
action increase with the size of the dose. Clinically, this is a critical issue
because the pharmacokinetics and pharmacody-namics of small doses of regular
and NPH insulins differ greatly from those of large doses. The delayed
absorption, dose-dependent duration of action, and variability of absorption (∼ 25%) of regu-lar human insulin frequently
results in a mismatching of insulin availability with need, and its use is
declining.
However,
short-acting, regular soluble insulin is the only type that should be
administered intravenously because the dilution causes the hexameric insulin to
immediately dissociate into mono-mers. It is particularly useful for
intravenous therapy in the manage-ment of diabetic ketoacidosis and when the
insulin requirement is changing rapidly, such as after surgery or during acute
infections.
3. Intermediate-acting and long-acting insulins
a. NPH (neutral protamine Hagedorn, or isophane) insulin—-NPH insulin is an
intermediate-acting insulin whose absorption and onset of action are delayed by
combining appropriate amounts of insulin and protamine so that neither is
present in an uncom-plexed form (“isophane”). After subcutaneous injection,
prote-olytic tissue enzymes degrade the protamine to permit absorption of
insulin. NPH insulin has an onset of approximately 2–5 hours and duration of
4–12 hours (Figure 41–5); it is usually mixed with regular, lispro, aspart, or
glulisine insulin and given two to four times daily for insulin replacement.
The dose regulates the action profile; specifically, small doses have lower,
earlier peaks and a short duration of action with the converse true for large
doses. The action of NPH is highly unpredictable, and its vari-ability of
absorption is over 50%. The clinical use of NPH is waning because of its
adverse pharmacokinetics combined with the availability of long-acting insulin
analogs that have a more predictable and physiologic action.
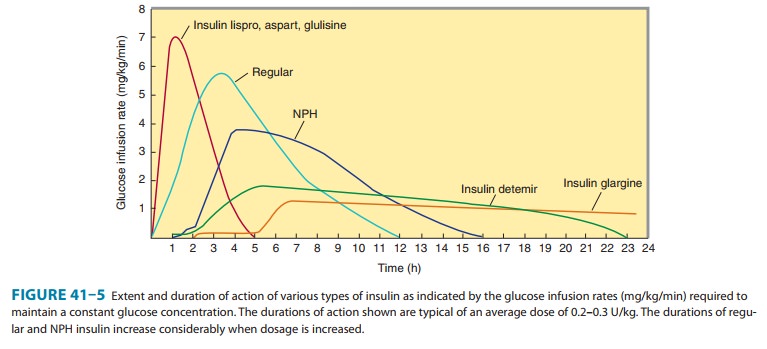
Insulin glargine—Insulin glargine is a soluble, “peakless” (ie,having a broad
plasma concentration plateau), long-acting insulin analog. This product was
designed to provide reproducible, con-venient, background insulin replacement.
The attachment of two arginine molecules to the B-chain carboxyl terminal and
substitu-tion of a glycine for asparagine at the A21 position created an analog
that is soluble in an acidic solution but precipitates in the more neutral body
pH after subcutaneous injection. Individual insulin molecules slowly dissolve
away from the crystalline depot and provide a low, continuous level of
circulating insulin. Insulin glargine has a slow onset of action (1–1.5 hours)
and achieves a maximum effect after 4–6 hours. This maximum activity is
main-tained for 11–24 hours or longer. Glargine is usually given once daily,
although some very insulin-sensitive or insulin-resistant individuals benefit
from split (twice a day) dosing. To maintain solubility, the formulation is
unusually acidic (pH 4.0), and insulin glargine should not be mixed with other
insulins. Separate syringes must be used to minimize the risk of contamination
and subse-quent loss of efficacy. The absorption pattern of insulin glargine
appears to be independent of the anatomic site of injection, and this drug is
associated with less immunogenicity than human insu-lin in animal studies.
Glargine’s interaction with the insulin recep-tor is similar to that of native
insulin and shows no increase in mitogenic activity in vitro. It has sixfold to
sevenfold greater bind-ing than native insulin to the insulin-like growth
factor-1 (IGF-1) receptor, but the clinical significance of this is unclear.
c. Insulin detemir—This
insulin is the most recently developedlong-acting insulin analog. The terminal
threonine is dropped from the B30 position and myristic acid (a C-14 fatty acid
chain) is attached to the terminal B29 lysine. These modifications pro-long the
availability of the injected analog by increasing both self-aggregation in
subcutaneous tissue and reversible albumin binding. Insulin detemir has the
most reproducible effect of the intermediate- and long-acting insulins, and its
use is associated with less hypoglycemia than NPH insulin. Insulin detemir has
a dose-dependent onset of action of 1–2 hours and duration of action of more
than 12 hours. It is given twice daily to obtain a smooth background insulin
level.
4. Mixtures of insulins—Because intermediate-acting
NPHinsulins require several hours to reach adequate therapeutic levels, their
use in diabetic patients usually requires supplements of rapid- or short-acting
insulin before meals. For convenience, these are often mixed together in the same
syringe before injection. Insulin lispro, aspart, and glulisine can be acutely mixed (ie, just before
injection) with NPH insulin without affecting their rapid absorption. However, premixed preparations have thus far been
unstable. To remedy this, intermediate insulins composed of isophane complexes
of protamine with insulin lispro and insulin aspart have been developed. These
intermediate insulins have been designated as “NPL” (neutral protamine lispro)
and “NPA” (neu-tral protamine aspart) and have the same duration of action as
NPH insulin. They have the advantage of permitting formulation as premixed
combinations of NPL and insulin lispro, and as NPA and insulin aspart, and they
have been shown to be safe and effec-tive in clinical trials. The Food and Drug
Administration (FDA) has approved 50%/50% and 75%/25% NPL/insulin lispro and
70%/30% NPA/insulin aspart premixed formulations. Additional ratios are
available abroad. Insulin glargine and detemir must be given as separate
injections. They are not miscible acutely or in a premixed preparation with any
other insulin formulation.
Premixed formulations
of 70%/30% NPH/regular continue to be available. These preparations have all
the limitations of regular insulin, namely, highly dose dependent pharmacokinetic
and pharmacodynamic profiles, and variability in absorption.
B. Insulin Production
Mass production of
human insulin and insulin analogs by recom-binant DNA techniques is carried out
by inserting the human or a modified human proinsulin gene into Escherichia coli or yeast and treating
the extracted proinsulin to form the insulin or insulin analog molecules.
C. Concentration
All
insulins in the USA and Canada are available in a concentra-tion of 100 U/mL
(U100). A limited supply of U500 regular human insulin is available for use in
rare cases of severe insulin resistance in which larger doses of insulin are
required.
Related Topics