Chapter: Microbiology and Immunology: Structure and Function Imune System
Cells of the Lymphoreticular System
Cells of the Lymphoreticular System
It is essential for the immune system to distinguish its own
molecules, cells, and organs (self) from those of foreign origin (nonself). The
innate immunity does this by express-ing germline-encoded pattern recognition
receptors on the surface of its cells, receptors that recognize structures on
potentially invasive microorganisms. The adaptive immunity, on the other hand,
makes use of somatically gen-erated epitope-specific T-cell receptors (TCRs)
and B-cell receptors (BCRs). These receptors are produced randomly and fresh
within each individual T and B lymphocytes by gene
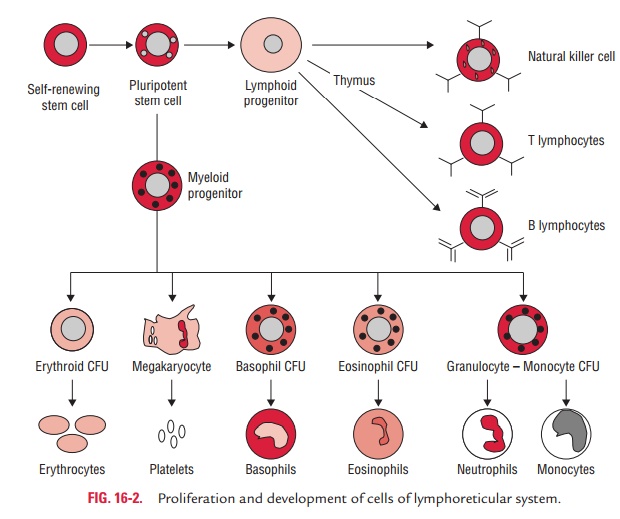
recombination prior to encounter with antigens (Fig. 16-2). The
cells involved in the adaptive immune responses are (a) lymphocytes, (b)
antigen-presenting cells (APCs), and (c)
effector cells that function to eliminate antigens.
Lymphocytes
The lymphocytes occupy a very special place among the leukocytes.
·
They participate in immune reactions due to their ability to
interact specifically with antigenic substances and to react to nonself
antigenic determinants.
·
They also contribute to the memory of the immune system.
The lymphocytes consist of heterogeneous populations of cells that
differ greatly from each other in terms of origin, lifespan, preferred areas of
settlement within the lymphoid organs, surface structure, and function. They
differentiate from stem cells in the fetal liver, bone marrow, and thy-mus into
two main functional classes: B cells and T cells. They are found in the
peripheral blood and in all lymphoid tissues.
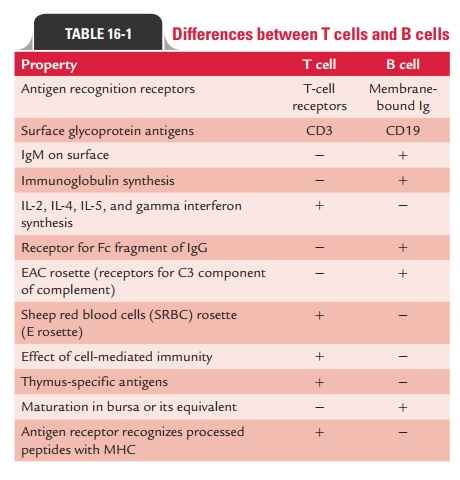
The lymphocytes are classified depending upon where they undergo
their development and proliferation: (a)
T lympho-cytes or T cells undergoing development in the thymus or (b) B lymphocytes, or B cells undergoing
development in the bone marrow. Differences between T cells and B cells are
summarized in Table 16-1.
◗ Thymus-derived cells
T lymphocytes, or T cells, are so designated because the thymus
plays a key role in their differentiation. They are the key play-ers in
adaptive immunity. They participate directly in immune responses as well as in
orchestrating and regulating activities of other cells.
·
T cells constitute 65–80% of the circulating pool of small
lymphocytes.
·
They are found in the inner subcortical regions but not in the
germinal centers of the lymph nodes.
·
They have a longer life span (months or years) than B lymphocytes.
·
They are stimulated to divide on exposure to certain mito-gens,
such as phytohemagglutinin or concavalin A, the T cells can be stimulated to
divide.
·
Most human T cells have receptors for sheep erythrocytes on their
surface and have the ability to form rosettes with them; this property is made
use of for identifying T cells in a mixed population of cells.
The T lymphocytes perform two important groups of func-tions as
follows:
Regulation of immune
responses: Regulatory function ismediated primarily by helper (CD41) T cells, which produce
interleukins.
Various effector functions: Effector functions are
mediatedprimarily by cytotoxic (CD81) T cells, which kill allografts, tumor cells,
and virus-infected cells. Depending on whether they have CD4 or CD8 proteins on
their surface, T cells are subdivided into two major groups: CD41 T cells and CD81 T cells. Mature T cells have
either CD4 or CD8 proteins, but never both.
CD41 T cells
CD4 cells are also known as helper T (Th) cells. They consti-tute
about 65% of peripheral T cells and are found mainly in the thymic medulla,
tonsils, and blood. CD4 displayed on the surfaces of these T cells recognize a
nonpeptide-binding portion of MHC class II molecules. Hence, CD41 T cells are restricted to
the recognition of pMHC class II complexes. Helper T lymphocytes are involved
in the induction and reg-ulation of immune responses. CD41 T cells perform follow-ing
helper functions:
·
They help B cells to be transformed into plasma cells.
·
They help CD81 T cells
to become activated cytotoxic T cells.
·
They help macrophages to mediate delayed type hypersen-sitivity
reactions.
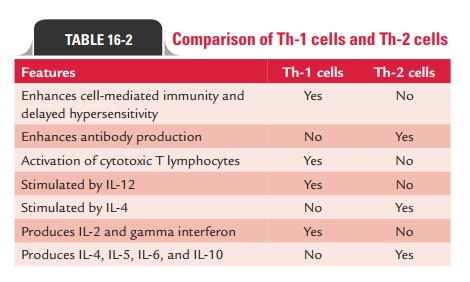
All these functions are mediated by Th-1 cells and Th-2 cells— the
two subpopulations of CD41 T cells:
·
The Th-1 cells activate cytotoxic T cells by producing IL-2.
·
They help in the development of hypersensitivity responses by
producing primarily IL-2 and gamma interferon.
·
The Th-2 cells perform B-cell helper function by producing
primarily IL-4 and IL-5.
The balance between Th-1 and Th-2 cells is regulated by gamma
interferon and IL-12. Gamma interferon inhibits the production of Th-2 cells,
whereas IL-12 increases the num-ber of Th-1 cells, thereby increasing host
defense against microorganisms that are controlled by a delayed
hypersensi-tivity reaction. Table 16-2 shows a comparison of Th-1 and Th-2
cells.
CD81 T cells
CD81 T cells are also known as cytotoxic T (Tc) and suppres-sor T (Ts)
cells. They account for approximately one-third of all mature CD31 cells. They are found mainly
in the human bone marrow and gut lymphoid tissue.
CD81 T glycoprotein displayed on the surfaces of these T cells
recognize a nonpeptide-binding portion of MHC class I molecules. Hence, CD81 T cells are restricted to
the recognition of pMHC class I complexes.
CD81 T cells perform mainly cytotoxic functions. They kill (a) virus-infected cells, (b) allograft cells, and (c) tumor cells. T-cell mediated
cytotoxicity is an apoptotic process that appears to be mediated by two
different pathways:
(i)One pathway involves the
release of proteins known as perforins, which insert themselves in the target
cell membranes forming channels. These channels allow the diffusion of enzymes
(granzymes, which are serine ester-ases) into the cytoplasm. The exact way in
which gran-zymes induce apoptosis has not been established, but
granzyme-induced apoptosis is Ca21-dependent.
(ii) The other pathway depends on signals delivered by the cytotoxic cell
to the target cell, which require cell-to-cell contact. This pathway is Ca21 independent.
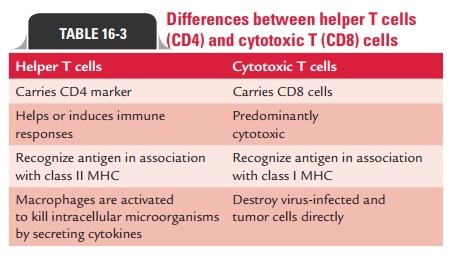
The ratio of CD41 and CD81 T cells is approximately 2:1 in normal human peripheral blood. This may be significantly altered in immunodeficiency diseases, autoimmune diseases, and other disorders. Differences between CD4 and CD8 T cells are summarized in Table 16-3.
Activation of T cells
Recognition of complex on the surface of APCs, such as mac-rophages
and dendritic cells, consisting of both the antigen and a class II MHC protein
by TCR present on T cells, is most important for activation of helper T cells.
Two signals are required to activate T cells:
·
The interaction of the antigen and the MHC protein with the
T-cell-receptor-specific antigen is the first signal required in the activation
of process. IL-1 secreted by the macrophages is also necessary for efficient
helper T-cell activation.
·
A costimulatory signal is the second signal required for activation
of T cells. In this signal, B7 protein present on the APC must interact with
CD28 protein on the helper T cells. Following the costimulatory signal, IL-2 is
produced by helper T cells, which is most crucial in producing a helper T cell
capable of performing their regulatory, effector, and memory functions.
After activation of the T cells, a new different protein called
CTLA-4 appears on the cell surface of T cells and binds to B7 by displacing
CD28. The interaction of CTLA-4 with B7 inhibits T-cell activation by blocking
IL-2 synthesis. This makes T cells to remain in a quiescent state and thereby
plays an important role in T-cell homeostasis. On the other hand, mutant T
cells that lack CTLA-4 and hence cannot be deactivated participate more
frequently in autoimmune diseases.
Memory T cells
Memory T cells, as the name suggests, confer host immunity with the
ability to respond rapidly and vigorously for many years after the initial
exposure to a microbe or other foreign substances. The memory produced against
a specific antigen shows the following characteristics:
1.
Memory cells live for many years or have the capacity to reproduce
them.
2.
A large number of memory cells are produced, and so secondary
response is enhanced and is greater than the primary response.
3.
Memory cells are activated by small quantity of antigens and
require less costimulation than do the naïve and unac-tivated T cells.
4.
Activated memory cells produce greater amounts of interleukins than
do naïve T cells when they are first activated.
T-cell receptor
T- cell receptor (TCR) for antigen consists of two polypep-tides:
alpha and beta. These two peptides are associated with CD3 proteins. Each T
cell has a unique TCR on its surface, thereby implying that hundreds of
millions of different T cells occur in each person. Activated T cells as well as
activated B cells produce large number of cells specific for those antigens.
T-cell alpha and beta polypeptides show many similarities to immunoglobulin
heavy chain in the following ways:
·
The genes coding for T-cell polypeptides are formed by
rearrangement of multiple regions of DNA.
·
There are V (variable), D (diversity), J (joining), and C
(con-stant) segments that rearrange to provide diversity, thereby resulting in
more than 107 different receptor proteins.
·
RAG-1 and RAG-2 are the two genes that encode the recombinase
enzymes that catalyze these gene rearrange-ments and are similar in T cells and
B cells.
·
T cells, however, differ from immunoglobulins in the following
ways:
·
T cells have two chains rather than four chains in immunoglobulins.
·
T cells recognize antigen only in conjunction with MHC proteins,
whereas immunoglobulins recognize free antigens.
Effect of superantigens on T cells
Certain proteins such as staphylococcal enterotoxins and toxic
shock syndrome toxins, and certain viral proteins, such as mouse mammary tumor
virus, are called superantigens. These are called “super” because they activate a
large number of helper T cells unlike “antigens”, which activate one or a few
helper cells.
The superantigens play a very important role in patho-genesis of
staphylococcal toxic shock syndrome caused by Staphylococcus aureus. In this condition, toxic shock syndrometoxin
produced by S. aureus binds directly
to class II MHC pro-teins without internal processing of the toxin.
Subsequently, this toxin interacts with variable component of the beta chain (Vb) of the T-cell receptor of
many T cells. The activation of T cells results in release of the interleukins,
IL-2 from the T cells and tumor necrosis factor (TNF) from macrophages. These
interleukins are responsible for many of the clinical presenta-tions observed
in toxin-mediated staphylococcal diseases.
Effector functions of T cells
T cells perform two important functions: (a) cytotoxicity and (b) delayed
hypersensitivity.
Cytotoxicity: Cytotoxicity activity of T
cells is required primar-ily to destroy virus-infected cells and tumor cells.
It also plays an important role in graft rejection. The cytotoxic T cells kill
the virus-infected cells:
a)
By inserting perforins and granzymes (degrading enzymes) into the
infected cell,
b)
By the Fas–Fas ligand (FasL) interaction, and
c)
By antibody-dependent cellular cytotoxicity (ADCC mechanism.
·
By inserting perforins and granzymes: Perforins are insertedinto
the cells, leading to formation of a channel through the membrane. This results
in the loss of cell contents and finally death of the cell. Granzymes are
proteins that degrade proteins in the cell membrane, which also results in loss
of cell contents. These enzymes also activate caspases that causes apoptosis,
resulting in cell death.
·
By the Fas–Fas ligand (FasL) interaction: Cytotoxic T cellskill
virus-infected cells by the FasL interaction. FasL is a pro-tein which is
expressed on the surface of many cells. When a cytotoxic TCR recognizes an
epitope on the surface of virus-infected cells, FasL appears on the cytotoxic T
cells. When Fas and FasL interact, it results in death or apoptosis of target
cells. NK cells can also kill target cells by FasL interaction.
·
By antibody-dependent cellular cytotoxicity (ADCC): Virus-infected cells can also
be killed by ADCC. In this process, target cells are killed by a combination of
IgG and phagocytic cells. The antibody bound to the surface of the infected
cells is rec-ognized by IgG receptor on the surface of phagocytic cells (e.g.,
macrophages, NK cells) and the infected cell is killed. After killing of the
virus-infected cells, the cytotoxic T cells are not damaged and can continue to
kill other cells infected with the same virus. However, the cytotoxic T cells
do not have any effect on free virus; they have effect only on virus-infected
cells.
The cytotoxic T cells kill the tumor cells by a
phenomenon called immune surveillance.
New antigens are usually developed on surfaceof many tumor cells. These
antigens bound to class I proteins are recognized by cytotoxic T cells, which
are activated to proliferate by IL-2. The resultant clone of cytotoxic T cells
can kill the tumor cells.
The cytotoxic T cells also play an important
role in graft rejec-tion. In this process, cytotoxic CD8 cells recognize the
class I MHC molecules on the surface of the foreign cells. Helper CD4 cells
recognize the foreign class II molecules on certain cells, such macrophages and
lymphocytes in the graft. The activated helper cells secrete IL-2, which
stimulates the cytotoxic cells to produce a clone of cells, which kills the
cells in the allograft.
Delayed hypersensitivity: The CD4 cells particularly
the Th-1subset cells and macrophages mediate the delayed hypersensitiv-ity
reactions against antigens of many intracellular pathogens. The CD4 cells
produce interleukins, such as gamma interferon, macrophage activation factor,
and macrophage inhibition fac-tor, which mediate delayed hypersensitivity reactions.
Th-1 cells produce IL-12-gamma interferon, which activates
macrophages and thereby enhances the ability of the macro-phages to kill Mycobacterium tuberculosis. The gamma
inter-feron, therefore, plays an important role in the ability of host immunity
to control infections caused by M.
tuberculosis, Listeriamonocytogenes,
and other intracellular microbes. A deficiency ofCMI makes the person highly
susceptible to infection by these microorganisms.
Regulatory functions of T cells
T cells play key role in regulating antibody production and in
suppression of certain immune responses.
1. Regulation of antibody production: Production of anti-bodies by B
cells may be (a) T-cell dependent,
requiring the participation of helper T cells (T-cell-dependent response), or (b) non-T-cell dependent
(T-cell-independent response).
In the
T-cell-dependent response, all the classes of immuno-globulins, such as IgG,
IgM, IgA, IgE, and IgD, are synthesized. The T-cell-dependent response produces
memory B cells.
In the non-T-cell dependent
response (T-cell-independent response), only IgM antibody is synthesized. This
response does not produce any memory cells. Hence, a secondary antibody
response does not occur. In this response, the multivalent macromolecules, such
as bacterial capsule polysaccharide are not effectively processed and presented
by APCs; hence these do not activate helper T cells. This is because
polysaccharides do not bind to class II MHC pro-teins, whereas peptide antigens
do.
2. Stimulation of helper and cytotoxic T cells to partici-pate in the CMI:
In CMI, the antigen is processed by mac-rophages and is presented in
conjunction with class II MHC molecules on the surface. These interact with the
receptor on the helper T cells, which is then activated to produce IL-2, a
T-cell growth factor that stimulates the specific helper and cytotoxic T cells
to grow and participate in the CMI.
3. Suppression of certain immune responses: T cells havebeen shown to
inhibit several immune-mediated diseases in animals. Regulatory T cells (TR),
also called suppressor T cells, is a subset of T cells and are associated with
the suppression of certain immune responses. TR cells also called suppressor T
cells are characterized by possessing CD25 marker and comprise 5–10% of the CD41 cells. The exact mechanism
by which the regulatory cells suppress the immune response is not known.
Imbalance in numbers or activity between CD4 and CD8 cells also leads to
impair-ment of the cellular immune response of the host.
◗
Bone marrow-derived cells
The bone marrow-derived lymphocytes are known as B lym-phocytes or
B cells. Plasma cells are derived from mature B cells. Both B cells and plasma
cells synthesize and secrete immunoglobulin.
B cells
B lymphocytes
or B cells are so designated because the bursa of Fabricius, a lymphoid organ
located close to the caudal end of the gut in birds, plays a key role in their
differentiation. A mammalian equivalent of the bursa is yet to be found. Here
the early stages of maturation of these lymphocytes occur in the bone marrow.
Origin of B cells: The clonal selection theory explains the
ori-gin of antibody formation. According to this postulation, each
immunologically competent B cell possesses receptor for either IgM or IgD that
can combine with one antigen or closely related antigens. After binding of the
antigen, the B cell is activated to proliferate and form a clone of cells.
Selected B cells are trans-formed to plasma cells that secrete antibodies
specific for the antigen. Plasma cells synthesize the immunoglobulin with the
same antigenic specificity as those carried by activated B cells. The same
clonal selection also occurs with T cells.
B cell
precursors, during embryogenesis, first proliferate and develop in the fetal
liver. From there, they migrate to the bone marrow, the main site of B-cell
maturation in the adults. Unlike T cells, they do not require the thymus for
maturation. The Pre-B cells have only m heavy chains in the cytoplasm but do not have
surface immunoglobulins and light chains. Pre-B cells are found in the bone
marrow, while B cells are found in the circulation. B cells mature in two
phases:
·
Antigen-independent phase, which consists of stem cells and pre-B
cells
·
Antigen-dependent phase, which consists of the cells, such as
activated B cells and plasma cells that proliferate on inter-actions of antigen
with B cells.
B cells possess surface IgM, which acts as a
receptor for antigen. Some B cells may also carry on their surface IgD as
receptor for the antigen. There are many other molecules expressed on the
surface of the B cells, which serve different functions. A few of them are B220,
class II MHC molecules, CR1 and CR2, CD40, etc.
Activation of B cells: Activation of B cells to
produce the fullrange of antibodies first requires recognition of the epitope
by the T-cell-antigen receptor and the production of IL-4 and IL-5 by the helper
T cells. In addition, it also requires other costimu-latory interactions of
CD28 on the T cells with B7 on the B cells. The CD28–B7 interaction is
essential to produce IL-2. It also includes CD40L on the T cells, which must
interact with CD40 on the B cells. The CD40L–CD40 interaction is essential for
class switching from IgM to IgG and for switching between other immunoglobulin
classes to take place.
Effector functions of B
cells: Production of many plasmacells is the end result of activation of B
cells. The plasma cells in turn produce large amounts of immunoglobulins
specific for the epitope of the antigen. Some activated B cells also produce
memory cells, which remain in a stage of quiescence for months or years. Most
memory B cells have surface IgG that acts as the antigen receptor, but some
even have surface IgM. These quiescent memory cells are activated rapidly on
reexposure to antigen. Memory T cells produce interleukins that facilitate
antibody production by the memory B cells. The presence of these cells is
responsible for the rapid appearance of antibody in the secondary immune
responses.
◗ Antigen-presenting cells
Antigen presenting cells (APCs) include (a) macrophages and (b)
dendritic cells.
Macrophages
The mononuclear phagocytic system consists of monocytes circulating
in the blood and macrophages in the tissues. The monocyte is considered a
leukocyte in transit through the blood, which becomes a macrophage when fixed
in a tissue. Monocytes and macrophages as well as granulocytes are able to
ingest par-ticulate matter (microorganisms, cells, inert particles) and for
this reason are said to have phagocytic functions. The phago-cytic activity is
greater in macrophages, particularly after activa-tion by soluble mediators
released during immune responses, than in monocytes. Differentiation of a
monocyte into a tissue macrophage involves a number of changes as follows:
a)
The cell enlarges 5–10 folds.
b)
Its intracellular organelles increase in number
and complexity.
c)
It acquires increased phagocytic ability.
d)
It produces higher levels of hydrolytic enzymes.
e)
It begins to secrete a variety of soluble factors.
Macrophage-like cells serve different functions
in different tissues and are named according to their tissue location. Examples
include (a) alveolar macrophages in
the lung, (b) histiocytes in
connective tissues, (c) Kupffer cells
in the liver, (d) mesangial cells in
the kidney, (e) microglial cells in
the brain, and (f) osteoclasts in the
bone.
For their participation in
the immune reaction, the macro-phages need to be stimulated and reach an
“activated state.”
·
Macrophages can be activated by various cytokines, compo-nents of
the bacterial cell wall, and mediators of the inflam-matory response.
·
Gamma interferon produced by helper T cells is a potent activator
of macrophages and is secreted by various cells in response to appropriate
stimuli. Bacterial lipopolysaccha-rides (endotoxin), bacterial peptidoglycan,
and bacterial DNA are the substances that also activate macrophages.
·
Activated macrophages are more potent than normal macro-phages in
many ways, such as having greater phagocytic abil-ity and increased ability to
kill ingested microbes. They are better APCs, and they activate T-cell response
in a more effec-tive manner. By secreting various cytotoxic proteins, they help
in eliminating a broad range of pathogens, including virus-infected cells,
tumor cells, and intracellular bacteria.
Functions of macrophages: Macrophages perform threemain
functions: (a) phagocytosis, (b) antigen presentation, and (c) cytokine production (Table 16-4).
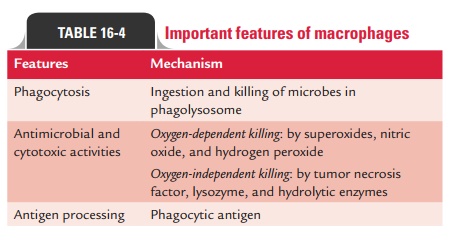
Phagocytosis: Phagocytosis of bacteria, viruses, and other
for-eign particles is the most important function of macrophages. The
macrophages on their cell surfaces have Fc receptors that interact with Fc
component of the IgG, thereby facilitating ingestion of the opsonized
organisms. They also have recep-tors for C3b, another important opsonin. After
ingestion, the phagosome containing the microbe fuses with a lysosome. The
microbe within the phagolysosome is killed by reactive oxygen, reactive
nitrogen compounds, and lysosomal enzymes.
Antigen presentation: After ingestion and degradation offoreign
materials, the fragments of antigen are presented on the macrophage cell
surface in conjunction with class II MHC proteins for interaction with the TCR
of CD41 helper T cells. Degradation of the foreign protein is stopped
following the association of antigen with the class II MHC proteins in the
cytoplasm. This is followed by transportation of the complex to the cell
surface by transporter proteins.
Cytokine production: Macrophages produce several cytokinesincluding
the IL-1, TNF, and IL-8. IL-1 plays an important role in activation of helper T
cells, while TNF plays as important mediator in inflammatory reactions. IL-8
attracts neutrophils and T cells to the site of infection.
Dendritic cells
Dendritic cells are so named because of their many long, nar-row
processes that resemble neuronal dendrites, which make them very efficient at
making contacts with foreign materials. They are primarily present in the skin
(e.g., Langerhans cells) and the mucosa, from where they migrate
Four types of dendritic cells are known: (i) Langerhans cells, (ii) interstitial dendritic cells, (iii) myeloid cells, and (iv) lymphoid dendritic cells. All these
cells constitutively express high levels of
both class II MHC molecules and members of the costimulatory B7
family. Following microbial invasion or during inflamma-tion, mature and
immature forms of Langerhans cells and inter-stitial dendritic cells migrate
into draining lymph nodes, where they make the critical presentation of antigen
to TH cells, which is required for the initiation of responses by
those key cells.
Follicular dendritic cells
Follicular dendritic cells are similar to the dendritic cells
except for their sites of presence and functions. These cells are pres-ent in
B-cell-containing germinal centers of the follicles in the spleen and lymph
nodes. These cells do not present antigen to helper T cells, but combine with
antigen–antibody complexes by Fc receptors found on their surfaces.
◗ Effector cells that function to eliminate antigens
Plasma cells
Plasma cells originate from terminally differentiated B cells.
Plasma cells are oval or egg-shaped structures characterized by a stellate
(star-like pattern) nucleus, nonstaining Golgi, and basophilic cytoplasm.
·
The main function of the plasma cells is to produce and secrete all
the classes of immunoglobulins into the fluids around the cells.
·
They secrete thousands of antibody molecules per second, which are
specific for the epitope of the antigen for a few days and then die.
·
They, however, do not express membrane immunoglobulins.
·
They divide very poorly, if at all, and are usually found in the
bone marrow and in the perimucosal lymphoid tissues.
·
They have a short lifespan of 30 days during which they produce
large quantities of immunoglobulins.
Natural killer cells
Natural killer (NK) cells are morphologically described as large
granular lymphocytes. These cells are called natural killer cells due to their
ability to kill certain virally infected cells and tumor cells without prior
sensitization. Their activities are not enhanced by exposure and are not
specific for any virus. NK cells comprise approximately 5–10% of peripheral
lymphocytes and are found in spleen and peripheral blood.
NK cells develop within the bone marrow and lack TCR, but possess
another set of receptors called killer activation recep-tors and killer
inhibition receptors. They also posses NK T cells, another subset of T cells,
which share some functional char-acteristics with NK cells. These NK T cells
unlike NK cells are stimulated by lipids, glycolipids, and hydrophobic peptides
presented by a nonclassical class I molecule CD1D and secrete large amounts of
cytokines, especially IL-4.
The main functions of the NK cells are to kill virus-infected cells
and tumors. They do so by secreting cytotoxins, such as perforins and granzymes
similar to those of cytotoxic T lym-phocytes and also by FasL-mediated
apoptosis. They kill the viruses without presence of specific antibodies but by
a mech-anism called ADCC. Both IL-12 and gamma interferons are potent
activators of NK cells.
Granulocytes
Granulocytes are a collection of white blood cells with seg-mented
or lobulated nuclei and granules in their cytoplasm, which are visible with
special stains. The granulocytes are classified as neutrophils, eosinophils, or
basophils on the basis of cellular morphology and cytoplasmic-staining
characteristics.
Both neutrophils and eosinophils are phagocytic, whereas basophils
are not. Eosinophils play an important role in defense against parasitic
infections, though their phagocytic role is significantly lower than
neutrophils. Basophils, on the other hand, are nonphagocytic granulocytes that
function by releasing pharmacologically active substances from their
cyto-plasmic granules. These substances play a major role in certain allergic
responses.
Mast cells are the other granulocytic cells that have a role in the
immune system. These cells are found in a wide variety of tissues, including
the skin, connective tissues of various organs, and mucosal epithelial tissue
of the respiratory, genitourinary, and digestive tracts. Like circulating
basophils, these cells have large numbers of cytoplasmic granules that contain
histamine and other pharmacologically active substances. Mast cells, together
with blood basophils, play an important role in the development of allergies.
Related Topics