Chapter: Essentials of Anatomy and Physiology: Body Temperature and Metabolism
Cell Respiration
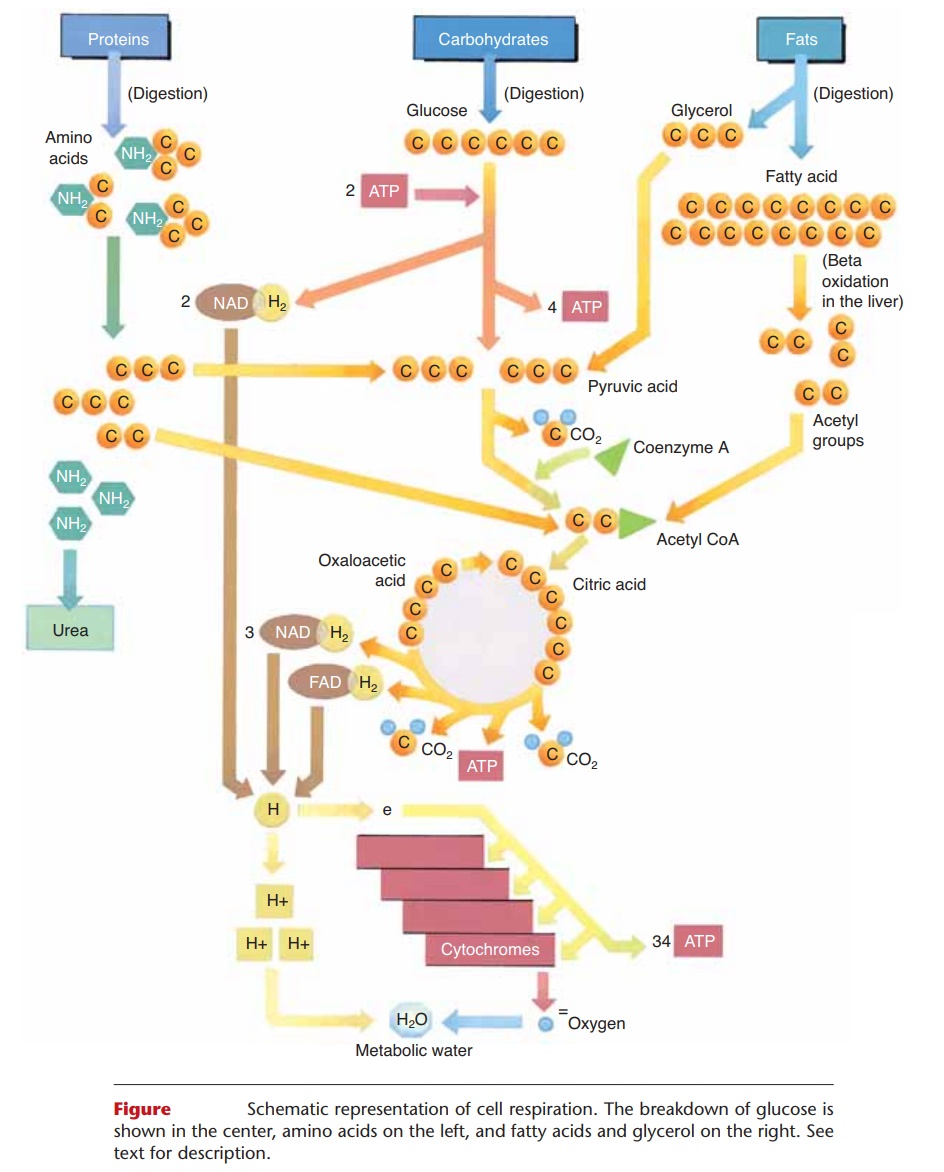
CELL RESPIRATION
You are already familiar with the summary reaction of cell respiration,
C6H12O6 + O2 → CO2 + H2O + ATP 1 Heat, (glucose)
the purpose of which is to produce ATP. Glucose con-tains potential energy, and when it is broken down to CO2 and H2O, this energy is released in the forms of ATP and heat. The oxygen that is required comes from breathing, and the CO2 formed is circulated to the lungs to be exhaled. The water formed is called metabolic water, and helps to meet our daily need for water. Energy in the form of heat gives us a body tem-perature, and the ATP formed is used for energy-requiring reactions. Synthesis of ATP means that energy is used to bond a free phosphate molecule to ADP (adenosine diphosphate). ADP and free phos-phates are present in cells after ATP has been broken down for energy-requiring processes.
The breakdown of glucose summarized here is not quite that simple, however, and involves a complex series of reactions. Glucose is taken apart “piece by piece,” with the removal of hydrogens and the split-ting of carbon–carbon bonds. This releases the energy of glucose gradually, so that a significant portion (about 40%) is available to synthesize ATP.
Cell respiration of glucose involves three major stages: glycolysis, the Krebs citric acid cycle, and the cytochrome (or electron) transport system. Although the details of each stage are beyond the scope of this book, we will summarize the most important aspects of each, and then relate to them the use of amino acids and fats for energy. This simple summary is depicted in Fig. 17–3.
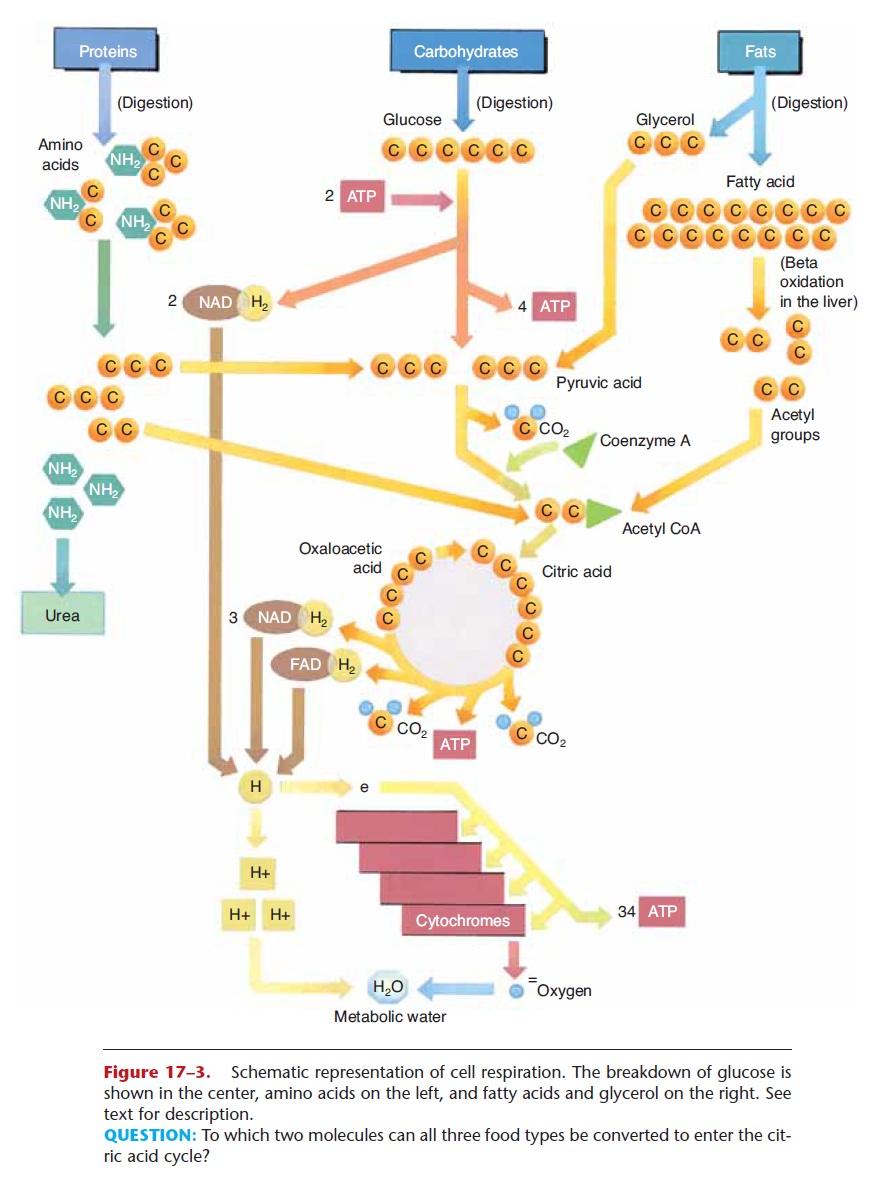
Figure 17–3. Schematic representation of cell respiration. The breakdown of glucose is shown in the center, amino acids on the left, and fatty acids and glycerol on the right. See text for description.
QUESTION: To which two molecules can all three food types be converted to enter the cit-ric acid cycle?
Glycolysis
The enzymes for the reactions of glycolysis are found in the cytoplasm of cells, and oxygen is not required (glycolysis is an anaerobic process). Refer now to Fig. 17–3 as you read the following. In glycolysis, a six-carbon glucose molecule is broken down to two three-carbon molecules of pyruvic acid. Two molecules of ATP are necessary to start the process. The energy they supply is called energy of activation and is neces-sary to make glucose unstable enough to begin to break down. As a result of these reactions, enough energy is released to synthesize four molecules of ATP, for a net gain of two ATP molecules per glucose molecule. Also during glycolysis, two pairs of hydrogens are removed by NAD, a carrier molecule that contains the vitamin niacin. Two NAD molecules thus become 2NADH2, and these attached hydrogen pairs will be transported to the cytochrome transport system (stage 3).
If no oxygen is present in the cell, as may happen in muscle cells during exercise, pyruvic acid is converted to lactic acid, which causes muscle fatigue. If oxygen is present, however, pyruvic acid continues into the next stage, the Krebs citric acid cycle (or, more simply, the Krebs cycle).
Krebs Citric Acid Cycle
The enzymes for the Krebs cycle (or citric acid cycle) are located in the mitochondria of cells. This second stage of cell respiration is aerobic, meaning that oxygen is required. In a series of reactions, a pyru-vic acid molecule is “taken apart,” and its carbons are converted to CO2. The first CO2 molecule is removed by an enzyme that contains the vitamin thiamine. This leaves a two-carbon molecule called an acetyl group, which combines with a molecule called coen-zyme A to form acetyl coenzyme A (acetyl CoA). As acetyl CoA continues in the Krebs cycle, two more carbons are removed as CO2, and more pairs of hydro-gens are picked up by NAD and FAD (another carrier molecule that contains the vitamin riboflavin). NADH2 and FADH2 will carry their hydrogens to the cytochrome transport system.
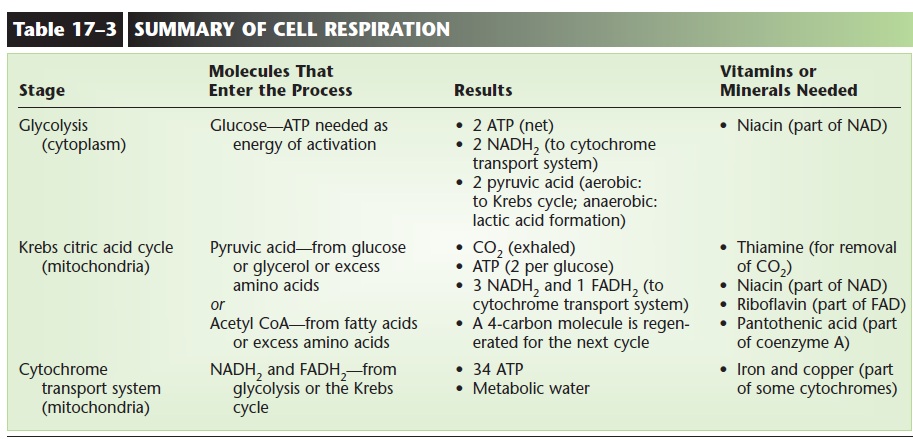
During the Krebs cycle, a small amount of energy is released, enough to synthesize one molecule of ATP (two per glucose). Notice also that a four-carbon mol-ecule (oxaloacetic acid) is regenerated after the forma-tion of CO2. This molecule will react with the next acetyl CoA, which is what makes the Krebs cycle truly a self-perpetuating cycle. The results of the stages of cell respiration are listed in Table 17–3. Before you continue, you may wish to look at that table to see just where the process has gotten thus far.
Cytochrome Transport System
Cytochromes are proteins that contain either iron or copper and are found in the mitochondria of cells. The pairs of hydrogens that were once part of glucose are brought to the cytochromes by the carrier mole-cules NAD and FAD. Each hydrogen atom is then split into its proton (H+ ion) and its electron. The electrons of the hydrogens are passed from one cytochrome to the next, and finally to oxygen. The reactions of the electrons with the cytochromes release most of the energy that was contained in the glucose molecule, enough to synthesize 34 molecules of ATP. As you can see, most of the ATP produced in cell respiration comes from this third stage.
Finally, and very importantly, each oxygen atom that has gained two electrons (from the cytochromes) reacts with two of the H+ ions (protons) to form water. The formation of metabolic water contributes to the necessary intracellular fluid, and also prevents acido-sis. If H+ ions accumulated, they would rapidly lower the pH of the cell. This does not happen, however, because the H+ ions react with oxygen to form water, and a decrease in pH is prevented.
The summary of the three stages of cell respiration in Table 17–3 also includes the vitamins and minerals that are essential for this process. An important over-all concept is the relationship between eating and breathing. Eating provides us with a potential energy source (often glucose) and with necessary vitamins and minerals. However, to release the energy from food, we must breathe. This iswhy we breathe. The oxygen we inhale is essential for the completion of cell respi-ration, and the CO2 produced is exhaled.
Proteins and Fats as Energy Sources
Although glucose is the preferred energy source for cells, proteins and fats also contain potential energy and are alternative energy sources in certain situations.
As you know, proteins are made of the smaller mol-ecules called amino acids, and the primary use for the amino acids we obtain from food is the synthesis of
In the liver, excess amino acids are deaminated, that is, the amino group (NH2) is removed. The remaining portion is converted to a molecule that will fit into the Krebs cycle. For exam-ple, a deaminated amino acid may be changed to a three-carbon pyruvic acid or to a two-carbon acetyl group. When these molecules enter the Krebs cycle, the results are just the same as if they had come from glucose. This is diagrammed in Fig. 17–3.
Fats are made of glycerol and fatty acids, which are the end products of fat digestion. These molecules may also be changed to ones that will take part in the Krebs cycle, and the reactions that change them usually take place in the liver. Glycerol is a three-carbon molecule that can be converted to the three-carbon pyruvic acid, which enters the Krebs cycle. In the process of beta-oxidation, the long carbon chains of fatty acids are split into two-carbon acetyl groups, which enter a later step in the Krebs cycle (see Fig. 17–3).
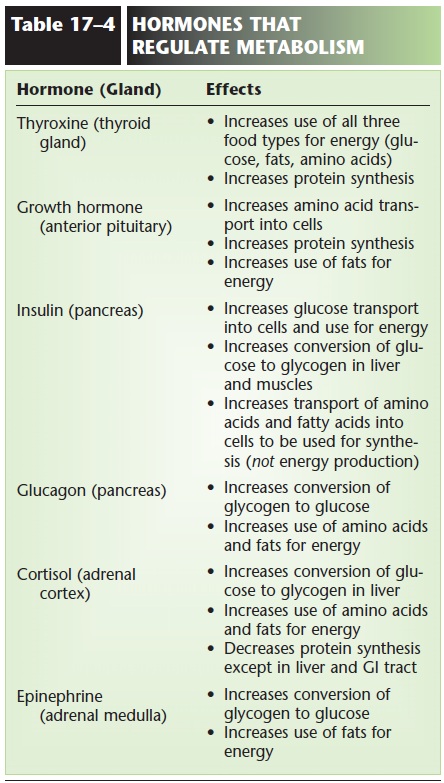
Both amino acids and fatty acids may be converted by the liver to ketones, which are two- or four-carbon molecules such as acetone and acetoacetic acid. Although body cells can use ketones in cell respira-tion, they do so slowly. In situations in which fats or amino acids have become the primary energy sources, a state called ketosis may develop; Ketosis. Excess amino acids may also be converted to glucose; this is important to supply the brain when dietary intake of carbohydrates is low. The effects of hormones on the metabolism of food are summarized in Table 17–4.
Energy Available from the Three Nutrient Types
The potential energy in food is measured in units called Calories or kilocalories. A calorie (lowercase “c”) is the amount of energy needed to raise the tem-perature of 1 gram of water 1°C. A kilocalorie or Calorie (capital “C”) is 1000 times that amount of energy.
One gram of carbohydrate yields about 4 kilocalo-ries. A gram of protein also yields about 4 kilocalories. A gram of fat, however, yields 9 kilocalories, and a gram of alcohol yields 7 kilocalories. This is why a diet high in fat is more likely to result in weight gain if the calories are not expended in energy-requiring activities.
You may have noticed that calorie content is part of the nutritional information on food labels. On such labels the term calorieactually means Calorie or kilo-calories but is used for the sake of simplicity.
Related Topics