Chapter: Mechanical : Automobile Engineering : Engine Auxiliary Systems Ignition System
Catalytic converter
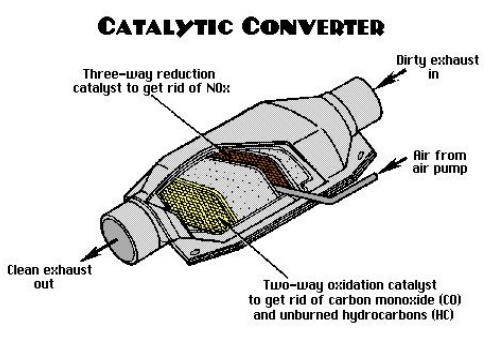
Catalytic converter
Catalytic converter is a vehicle emissions control device that
converts toxicpollutants in exhaust gas to less toxic pollutants by catalyzing
a redox reaction(oxidation or reduction). Catalytic converters are used in
internal combustion engines fueled by either petrol (gasoline) or diesel—
including lean burn engines.
The first widespread introduction of catalytic converters was
in the United Statesautomobile market. Manufacturers of 1975 model year
equipped gasoline-powered vehicles
with
catalytic converters to comply with the U.S. Environmental Protection Agency's
stricter regulation of exhaust emissions. These “two-way”
converters combined carbon monoxide (CO)
with
unburned hydrocarbons (HC) to produce carbon dioxide (CO2) and water (H2O). In
1981, two-way catalytic converters were rendered obsolete
by “three-way” converters that also reduceoxides of
nitrogen (NOx); however, two-way
converters are still used for lean burn engines.
Although catalytic converters are most commonly applied to
exhaust systems in automobiles, they are also used on electrical generators,
forklifts, mining equipment, trucks, buses, locomotives, motorcycles, and
airplanes. They are also used on some wood stoves to control emissions. This is
usually in response to government regulation, either through direct
environmental regulation or through health and safety regulations.
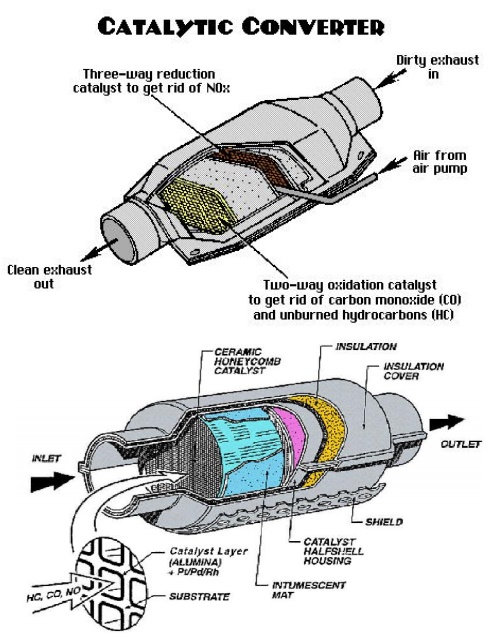
Construction
of a catalytic converter;
The catalyst support or substrate. For automotive catalytic
converters, the core is usually a ceramic monolith with a honeycomb structure.
Metallic foil monoliths made of Kanthal (FeCrAl) are used in applications where
particularly high heat resistance is required Either material is designed to
provide a large surface area. The cordierite ceramic substrate used in most
catalytic converters was invented by Rodney Bagley, Irwin Lachman andRonald
Lewis at Corning Glass, for which they were inducted into the National
Inventors Hall of Fame in 2002.
The washcoat. A washcoat is a carrier for the catalytic
materials and is used to disperse the materials over a large surface area.
Aluminum oxide, titanium dioxide, silicon dioxide, or a mixture of silica and
alumina can be used. The catalytic materials are suspended in the washcoat
prior to applying to the core. Washcoat materials are selected to form a rough,
irregular surface, which greatly increases the surface area compared to the
smooth surface of the bare substrate. This in turn maximizes the catalytically
active surface available to react with the engine exhaust. The coat must retain
its surface area and prevent sintering of the catalytic metal particles even at
high temperatures
The catalyst itself is most often a mix of precious metals.
Platinum is the most active catalyst and is widely used, but is not suitable
for all applications because of unwanted additional reactions and high cost.
Palladium and rhodium are two other precious metals used. Rhodium is used as a
reduction catalyst, palladium is
used as an oxidation catalyst, and
platinum is used
both for reduction and oxidation.
Cerium, iron, manganese and nickel are
also used, although each
has limitations. Nickel is not legal for use in the European Union
because of its reaction with carbon monoxide into toxic nickel tetracarbonyl.[citation needed] Copper
can be used everywhere except North
America,[clarification needed]where its use is illegal because of the formation
of toxic dioxin .[citation needed]
Related Topics