Auto-catalysis, Negative Catalysis, Promoters and Poisons - Catalysis | Engineering Chemistry: Surface Chemistry and Catalysis
Chapter: Engineering Chemistry: Surface Chemistry and Catalysis
Catalysis
Catalysis
When hydrogen and oxygen gases are kept in contact
with each other, no observable reaction occurs. If we add a small piece of
platinum gauge in the mixture of these gases, the reaction occurs readily. Here
platinum gauge speeds up the reaction and is called a catalyst.
A
catalyst is a substance which changes the rate of a reaction but remains
chemically unchanged at the end of the reaction.
The phenomenon of change of reaction rate by
addition of a substance which itself remains unchanged chemically is called catalysis. The following are some more
examples of catalysis:
(i) Decomposition of potassium chlorate occurs at
high temperature. If a small amount of the manganese dioxide is added, the
decomposition occurs at much lower temperature. Here, manganese dioxide acts as
catalyst.
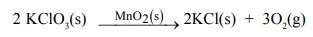
(ii) The evolution of hydrogen by the reaction
between zinc and hydrochloric acid is catalysed by Cu2+(aq) ions.
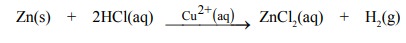
(iii) The oxidation of hydrogen chloride gas by
oxygen occurs more quickly if the gases are passed over cupric chloride.

Auto-catalysis
In certain reactions, one of the products of the
reaction acts as the catalyst. For example, the oxidation of oxalic acid by
acidified potassium permanganate occurs as
2KMnO4(aq) + 3H2SO4(aq)
+ 5(COOH)2(aq) → K2SO4(aq)
+ 2MnSO4(aq) + 8H2O( ) + 10 CO2(g)
At room temperature, the reaction is quite slow in
the beginning. Gradually it becomes fast due to the catalytic action of Mn2+
ions which are one of the products as MnSO4 in the reaction.
The phenomenon in which one of the products of a
reaction acts as a catalyst is known as auto-catalysis.
Negative Catalysis
Some catalysts retard a reaction rather than speed
it up. They are known as negative catalysts. For example :
(i) Glycerol
retards the decomposition of hydrogen peroxide.
(ii) Phenol
retards the oxidation of sulphurous acid.
Promoters and Poisons
Certain substances increase or decrease the
activity of the catalyst, although, by themselves they do not show any
catalytic activity.
The
substances which increase the activity of a catalyst are called promoters and those which decrease the
activity of a catalyst are called poisons.
For
example :
(i) In Haber’s process for the manufacture of
ammonia, the catalytic activity of iron is enhanced by molybdenum which acts as
promoter.
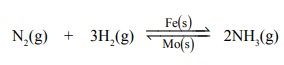
(ii) Copper promotes the catalytic activity of
nickel during hydrogenation of oils.
(iii) In Haber’s process the catalyst iron is
poisoned by hydrogen sulphide H2S.
(iv) In contact process for the manufacture of sulphuric
acid, the catalyst platinum is poisoned by even the traces of arsenious oxide
As2O3.
Related Topics