Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Capillary Electrophoresis Methods
Capillary Electrophoresis Methods
There are several
different forms of capillary electrophoresis, each of which has its particular advantages. Several of these methods
are briefly described in this section.
Capillary Zone Electrophoresis
The simplest form of capillary electrophoresis is capillary zone electrophoresis (CZE). In CZE the capillary tube is filled with a buffer solution and, after loading the sample, the ends of the capillary tube are placed in reservoirs containing additional buffer solution.
Under normal conditions, the end of the capillary containing the sample is the anode, and solutes migrate toward the cathode at a velocity
determined by their
electrophoretic mobility and the electroosmotic flow. Cations elute
first, with smaller,
more highly charged
cations elut- ing before
larger cations with
smaller charges. Neutral
species elute as a single
band. Finally, anions are
the last species
to elute, with
smaller, more negatively charged an- ions being
the last to elute.
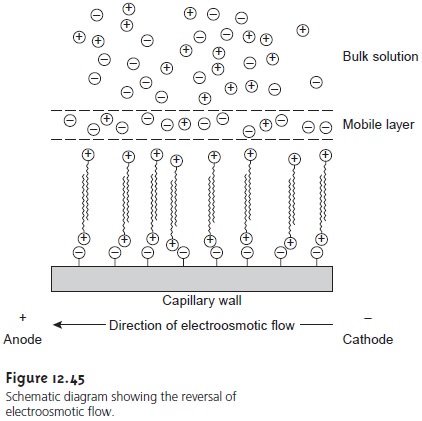
The direction of electroosmotic flow and, therefore, the order of elution in CZE
can be reversed. This is accomplished by adding an alkylammonium salt to the buffer solution. As shown
in Figure 12.45,
the positively charged
end of the
alkyl- ammonium ion binds
to the negatively charged silanate ions
on the capillary’s walls. The alkylammonium ion’s
“tail” is hydrophobic and associates with the tail of an- other alkylammonium ion. The result is a layer
of positive charges
to which anions in the buffer solution
are attracted. The migration of these solvated
anions toward the anode
reverses the electroosmotic flow’s direction. The order of elution in this
case is exactly the opposite
of that observed
under normal conditions.
Capillary zone electrophoresis also can be accomplished without
an electroos- motic flow by coating
the capillary’s walls
with a nonionic reagent. In the absence
of electroosmotic flow only
cations migrate from
the anode to the cathode. Anions elute into the source reservoir while neutral species
remain stationary.
Capillary zone electrophoresis provides effective separations of any charged species, including inorganic anions
and cations, organic
acids and amines,
and large biomolecules such as proteins.
For example, CZE has been used to separate a mix-
ture of 36 inorganic and organic ions in less than 3 minutes.17 Neutral species, of course,
cannot be separated.
Micellar Electrokinetic Capillary Chromatography
One
limitation to CZE
is its in- ability to separate neutral species. Micellar electrokinetic
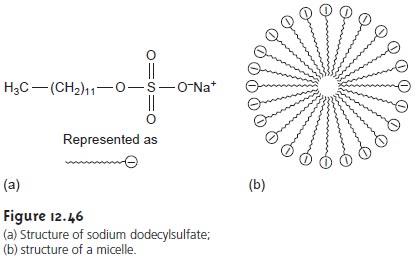
chromatography (MEKC) overcomes this limitation by adding a surfactant, such as sodium
dodecyl- sulfate (Figure 12.46a) to the buffer solution. Sodium
dodecylsulfate, (SDS) has a
long-chain hydrophobic “tail”
and an ionic functional group,
providing a negatively charged “head.”
When the concentration of SDS is sufficiently large, a micelle forms. A micelle consists of an agglomeration of 40–100 surfactant molecules in which the hydrocarbon tails
point inward, and the negatively charged heads point outward (Figure 12.46b).
Because micelles are
negatively charged, they
migrate toward the
cathode with a velocity less than the electroosmotic
flow velocity. Neutral species partition them-
selves between the
micelles and the
buffer solution in much the
same manner as they
do in HPLC. Because there is a partitioning between two phases, the term “chromatography” is used. Note that
in MEKC both phases are “mobile.”
Capillary Gel Electrophoresis
In capillary
gel electrophoresis (CGE) the capil- lary tubing is filled
with a polymeric gel. Because
the gel is porous, solutes
mi- grate through
the gel with a velocity
determined both by their electrophoretic mobility and their size.
The ability to effect a separation based
on size is useful when the solutes have similar
electrophoretic mobilities. For example, fragments of DNA of varying
length have similar
charge-to-size ratios, making
their separa- tion by CZE difficult. Since the DNA fragments are of different size, a CGE sepa-
ration is possible.
The capillary used for CGE is usually
treated to eliminate
electroosmotic flow, thus preventing the gel’s extrusion from the capillary tubing. Samples are injected
electrokinetically because the gel provides
too much resistance for hydrodynamic
sampling. The primary application of CGE is the separation of large biomolecules, including DNA
fragments, proteins, and
oligonucleotides.
Capillary Electrochromatography
Another approach to
separating neutral species is capillary
electrochromatography (CEC). In this technique the capillary tubing
is packed with 1.5–3-μm silica particles coated
with a bonded,
nonpolar stationary phase. Neutral
species separate based
on their ability
to partition between
the sta- tionary phase and the buffer solution
(which, due to electroosmotic flow, is the mo-
bile phase). Separations are similar
to the analogous HPLC separation, but without the need for high-pressure pumps. Furthermore, efficiency in CEC is better than in
HPLC, with shorter analysis times.
Related Topics