Chapter: Modern Analytical Chemistry: Calibrations, Standardizations, and Blank Corrections
Calibrating Signals
Calibrating Signals
Signals are measured
using equipment or instruments that must be properly cali- brated if Smeas
is to be free of determinate errors. Calibration is accomplished against a standard, adjusting Smeas until
it agrees with
the standard’s known
signal. Several common examples of calibration are
discussed here.
When the signal
is a measurement of mass,
Smeas is determined with
an analyti- cal balance.
Before a balance
can be used, it must be calibrated against a reference weight meeting standards established by either the
National Institute for
Standards and Technology or the American Society for Testing
and Materials. With
an elec- tronic balance
the sample’s mass is determined by the current
required to generate an upward electromagnetic force
counteracting the sample’s downward gravita- tional force. The balance’s calibration procedure invokes
an internally programmed calibration routine
specifying the reference
weight to be used. The reference weight is
placed on the balance’s weighing
pan, and the relationship between
the displace- ment of the weighing
pan and the counteracting current
is automatically adjusted.
Calibrating a balance, however, does not eliminate all sources
of determinate error. Due to the buoyancy of air, an object’s weight
in air is always lighter
than its weight in vacuum. If there is a difference between the density
of the object
being weighed and the density of the weights
used to calibrate the balance, then a correc- tion to the object’s weight must be made.
An object’s true
weight in vacuo,
Wv, is related to its weight
in air, Wa, by the equation
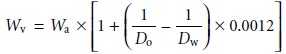
where Do is the object’s
density, Dw is the density of the calibration weight, and 0.0012 is the density
of air under normal laboratory conditions (all densities
are in units of g/cm3). Clearly
the greater the difference between
Do and Dw the more seri- ous
the error in the object’s
measured weight.
The buoyancy correction for a solid
is small, and frequently ignored.
It may be significant, however, for
liquids and gases
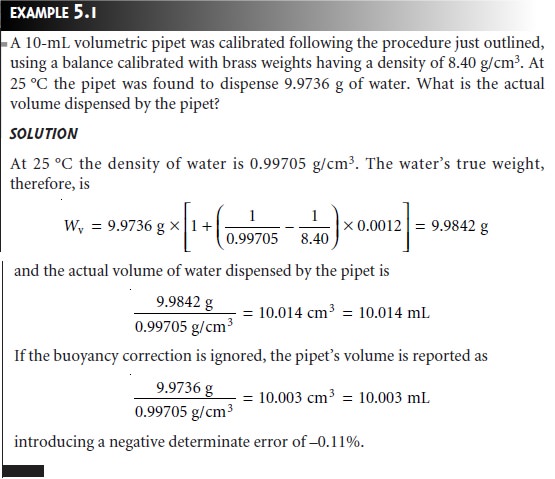
of low density. This is particularly important when calibrating glassware. For example, a
volumetric pipet is calibrated by carefully
filling the pipet
with water to its calibration mark, dispensing the water
into a tared beaker and determining the mass of water transferred. After correcting for the buoyancy of air, the density of water is used to calculate the volume of water
dispensed by the pipet.
Balances and volumetric glassware are examples of laboratory equipment. Lab- oratory
instrumentation also must be calibrated
using a standard providing a known
response. For example,
a spectrophotometer’s accuracy
can be evaluated by measuring the absorbance of a carefully prepared solution of 60.06 ppm K2Cr2O7 in 0.0050 M H2SO4, using
0.0050 M H2SO4 as a reagent
blank. The spectrophotome-
ter is considered calibrated if the resulting
absorbance at a wavelength of 350.0 nm is 0.640 ± 0.010 absorbance units. Be sure to read and carefully
follow the calibra- tion instructions provided with
any instrument you
use.
Related Topics