Chapter: Biochemistry: Water: The Solvent for Biochemical Reactions
Buffers
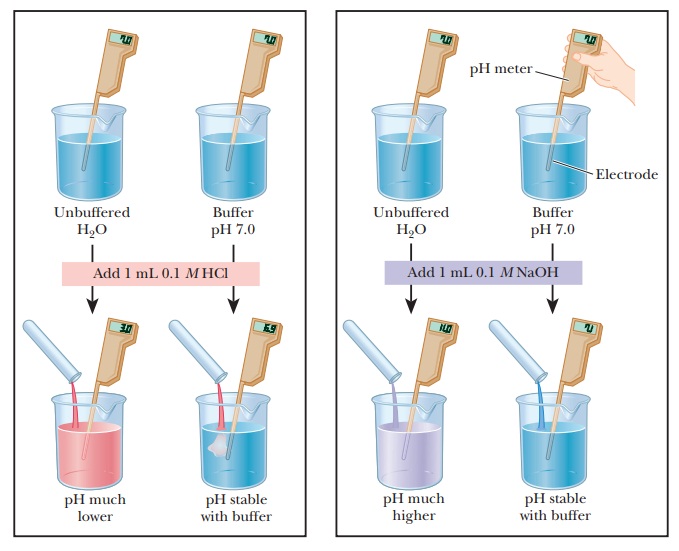
Buffers
A buffer is something that resists
change. In terms of acid and base chemistry, a buffer solution tends to resist change in pH when small to moderate
amounts of a strong acid or strong base are added. A buffer solution consists
of a mixture of a weak acid and its conjugate base.
How do buffers work?
Let us
compare the changes in pH that occur on the addition of equal amounts of strong
acid or strong base to pure water at pH 7 and to a buffer solution at pH 7. If
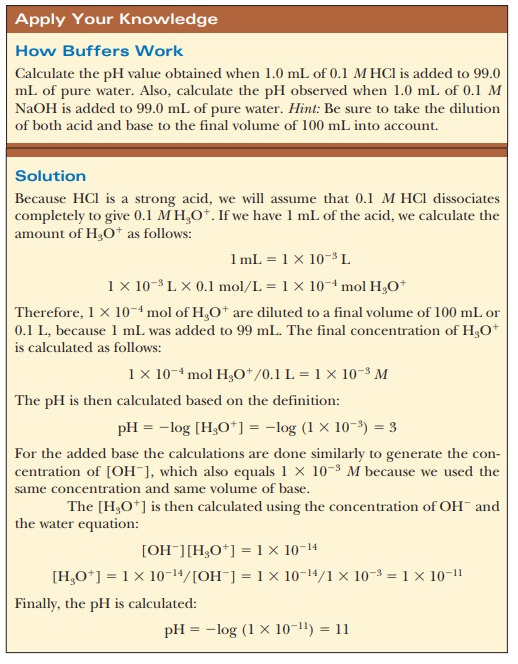
1.0 mL of 0.1 M HCl is added to 99.0
mL of pure water, the pH drops drastically. If the same experiment is conducted
with 0.1 M NaOH instead of 0.1 M HCl, the pH rises drastically (Figure
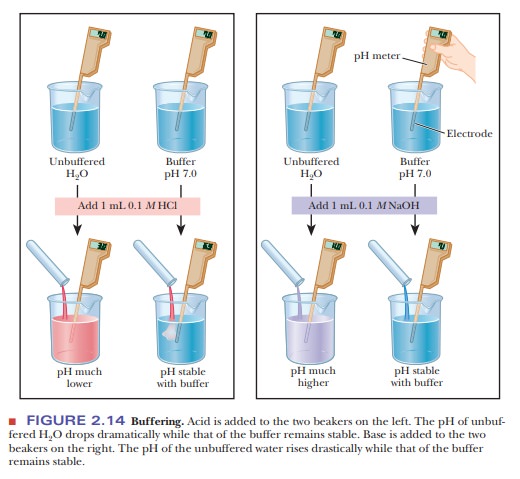
2.14).
The
results are different when 99.0 mL of buffer solution is used instead of pure
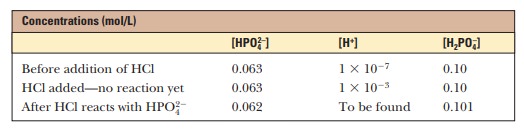
water. A solution that contains the monohydrogen phosphate and dihydrogen
phosphate ions, HPO42- and H2PO4-, in
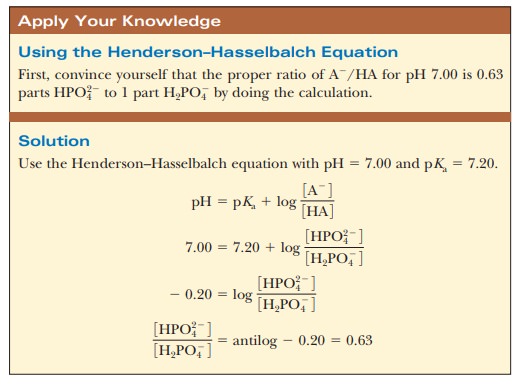
suitable proportions can serve as such a buffer. The Henderson–Hasselbalch
equation can be used to calculate the HPO42-/H2PO4- ratio
that corresponds to pH 7.0.
For
purposes of illustration, let us consider a solution in which the
concen-trations are [HPO42-] = 0.063 M and [H2PO4-] = 0.10 M; this gives the conjugate base/weak
acid ratio of 0.63 seen previously. If 1.0 mL of 0.10 M HCl is added to 99.0 mL of the buffer, the reaction
[HPO42-] 1 H+ ßà H2PO4-
takes
place, and almost all the added H+ will be used up. The
concentrations of [HPO42-] and [H2PO4-] will
change, and the new concentrations can be calculated.
The new
pH can then be calculated using the Henderson-Hasselbalch
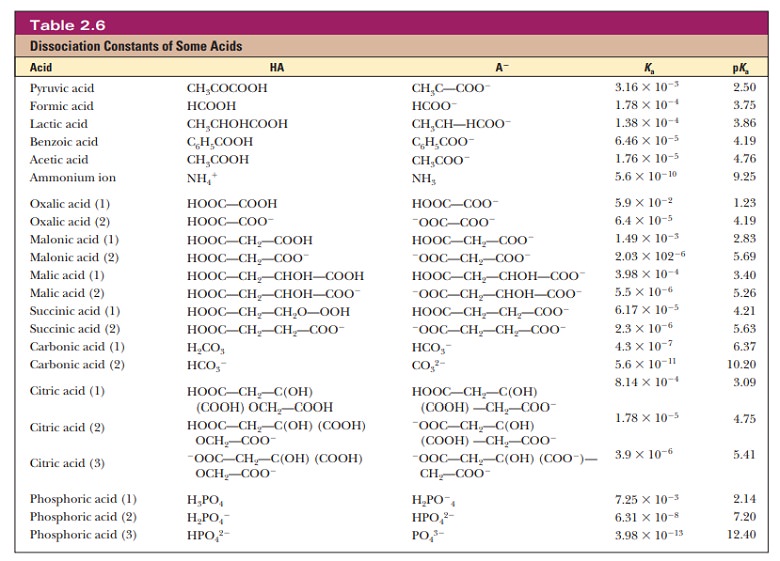
equation and the phosphate ion concentrations. The appropriate pKa is 7.20
(Table 2.6).

The new
pH is 6.99, a much smaller change than in the unbuffered pure water (Figure
2.14). Similarly, if 1.0 mL of 0.1 M
NaOH is used, the same reaction takes place as in a titration:
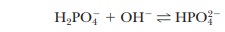
Almost
all the added OH- is used up, but a small
amount remains. Because this buffer is an aqueous solution, it is still true
that Kw= [H+][OH-]. The
increase in hydroxide ion concentration implies that the hydrogen ion
concentration decreases and that the pH increases. Use the
Henderson–Hasselbalch equation to calculate the new pH and to convince yourself
that the result is pH 5 7.01, again a much smaller change in pH than
took place in pure water (Figure 2.14). Many biological reactions will not take
place unless the pH remains within fairly narrow limits, and, as a result,
buffers have great practical importance in the biochemistry laboratory.
How do we choose a buffer?
A
consideration of titration curves can give insight into how buffers work
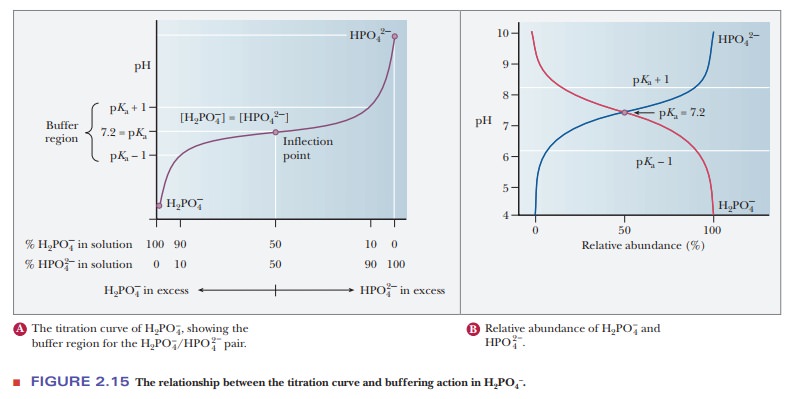
(Figure 2.15a). The pH of a sample being titrated changes very little in the vicinity
of the inflection point of a titration curve. Also, at the inflection point,
half the amount of acid originally present has been converted to the conjugate
base. The second stage of ionization of phosphoric acid,
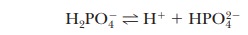
was the
basis of the buffer just used as an example. The pH at the inflection point of
the titration is 7.20, a value numerically equal to the pKa of the dihydrogen phosphate ion. At this pH, the solution contains
equal concentrations of the dihydrogen phosphate ions and monohydrogen
phosphate ions, the acid and base forms. Using the Henderson–Hasselbalch
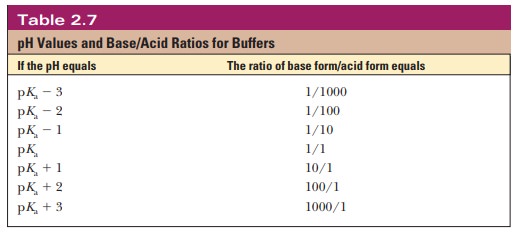
equation, we can calculate the ratio of the conjugate base form to the
conjugate acid form for any pH when we know the pKa. For example, if we choose a pH of 8.2 for a buffer composed of H2PO4- and HPO42-, we can
solve for the ratio
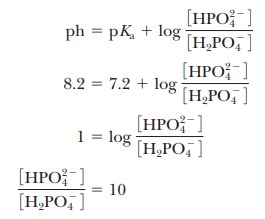
Thus,
when the pH is one unit higher than the pKa, the
ratio of the conjugate base form to the conjugate acid form is 10. When the pH
is two units higher than the pKa, the
ratio is 100, and so on. Table 2.7 shows this relationship for several
increments of pH value.
A buffer
solution can maintain the pH at a relatively constant value because of the
presence of appreciable amounts of both the acid and its conjugate base. This
condition is met at pH values at or near the pKa of the acid. If OH+ is added, an appreciable
amount of the acid form of the buffer is present in solu-tion to react with the
added base. If H+ is added, an appreciable
amount of the basic form of the buffer also is present to react with the added
acid.
The H2PO4/HPO4- pair is suitable as a buffer near pH 7.2, and the CH3COOH/CH3COO- pair is suitable as a buffer near pH 4.76. At pH values below the pKa, the acid form predominates, and at pH values above the pKa, the basic form predominates. The plateau region in a titration curve, where the pH does not change rapidly, covers a pH range extending approximately one pH unit on each side of the pKa. Thus, the buffer is effective within a range of about two pH units (Figure 2.15b).
In many
biochemical studies, a strict pH range must be maintained in order for the
experiment to be successful. Using our knowledge of the range of an effective
buffer compared to its pKa, we can
select an appropriate buffer. If we were doing an experiment and needed the pH
to be 7.2, we might select the H2PO4/HPO4- pair to
be our buffer. If we wanted a pH near 9.0, we would look at tables of buffers
to find one with a pKa close
to nine. The following Biochemical Connections box goes into greater detail on
buffer selection.
The condition that a buffer contains appreciable amounts of both a weak acid and its conjugate base applies both to the ratio of the two forms and to the absolute amount of each present in a given solution. If a buffer solution contained a suitable ratio of acid to base, but very low concentrations of both, it would take very little added acid to use up all of the base form, and vice versa. A buffer solution with low concentrations of both the acid and base forms is said to have a low buffering capacity. A buffer that contains greater amounts of both acid and base has a higher buffering capacity.
How do we make buffers in the laboratory?
When we
study buffers in theory, we often use the Henderson2Hasselbalch
equation and do many calculations concerning ratios of conjugate base form to
conjugate acid form. In practice, however, making a buffer is much easier. To
have a buffer, all that is necessary are the two forms of the buffer present in
the solution at reasonable quantities. This situation can be obtained by adding
predetermined amounts of the conjugate base form (A-) to the
acid form (HA), or we could start with one and create the other, which is how
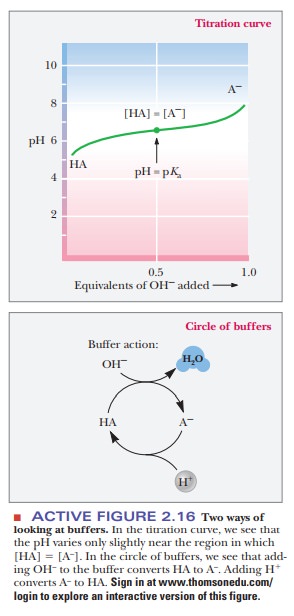
it is usually done in practice. Remember that HA and A- are
interconverted by adding strong acid or strong base (Figure 2.16). To make a
buffer, we could start with the HA form and add NaOH until the pH is correct,
as determined by a pH meter. We could also start with A- and add
HCl until the pH is correct. Depending on the relationship of the pH we desire
to the pKa of the
buffer, it may be more convenient to start with one than the other. For
example, if we are making an acetic acid/acetate buffer at pH 5.7, it would
make more sense to start with the A- form
and to add a small amount of HCl to bring the pH down to 5.7, rather than to
start with HA and to add much more NaOH to bring the pH up past the pKa.
Are naturally occurring pH buffers present in living organisms?
Up until
now, we have been considering buffers from the perspective of a chemist trying
to control an experiment. However, the real importance of buffers is that they
are critical to life. Buffer systems in living organisms and in the laboratory
are based on many types of compounds. Because physiological pH in most
organisms stays around 7, it might be expected that the phosphate buffer system
would be widely used in living organisms. This is the case where phosphate ion
concentrations are high enough for the buffer to be effective, as in most
intracellular fluids.
The H2PO4/HPO4- pair is
the principal buffer in cells. In blood, phosphate ion levels are inadequate for
buffering, and a different system operates.
The
buffering system in blood is based on the dissociation of carbonic acid (H2CO3):
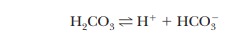
where
the pKa of H2CO3 is
6.37. The pH of human blood, 7.4, is near the end of the buffering range of
this system, but another factor enters into the situation.
Carbon
dioxide can dissolve in water and in water-based fluids, such as blood. The
dissolved carbon dioxide forms carbonic acid, which, in turn, reacts to produce
bicarbonate ion:

At the
pH of blood, which is about one unit higher than the pKa of carbonic acid, most of the dissolved CO2 is
present as HCO3-. The CO2 being
transported to the lungs to be expired takes the form of bicarbonate ion. A
direct relationship exists between the pH of the blood and the pressure of
carbon dioxide gas in the lungs. The properties of hemoglobin, the
oxygen-carrying protein in the blood, also enter into the situation.
The
phosphate buffer system is common in the laboratory (in vitro, outside the living body) as well as in living organisms (in vivo). The buffer system based on
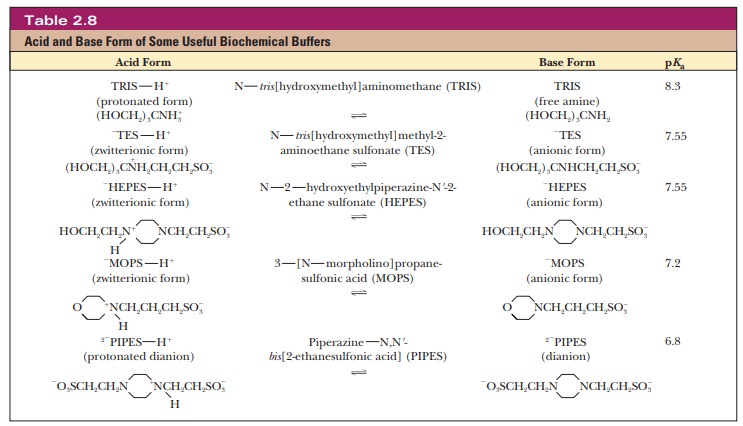
TRIS [tris(hydroxymethyl)aminomethane]
is also widely used in vitro. Other buffers that have come into wide use more
recently are zwitterions, which are
compounds that have both a positive charge and a negative charge. Zwitterions
are usually considered less likely to interfere with biochemical reactions than
some of the earlier buffers (Table 2.8).
Most
living systems operate at pH levels close to 7. The pKa values of many functional groups, such as the carboxyl and amino
groups, are well above or well below this value. As a result, under
physiological conditions, many important biomolecules exist as charged species
to one extent or another. The practical
Summary
Buffer solutions are characterized by their
tendency to resist pH change when small amounts of strong acid or strong base
are added.
Buffers work because the concentration of the
weak acid and base is kept in the narrow window of the acid titration curve.
Many experiments must have a buffered system to
keep a stable pH.
Many
physiological buffers, such as the bicarbonate blood buffer or the phosphate
buffer, help maintain physiological pH.
Related Topics