Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Brief history of periodic classification
Brief history of periodic classification
More than one hundred and nine elements are known today,. The periodic
table of elements is an important landmark in the history of chemistry. It
would be difficult to study individually the chemistry of all the elements and
their numerous compounds. The periodic table provides a systematic and
extremely useful framework for organizing a lot of information available on the
chemical behaviour of the elements into a few simple and logical patterns. This
gave rise to the necessity of classifying the elements into various groups or
families having similar properties. This classification has resulted in the
formulation of periodic table. Periodic table may be defined as the
arrangements of various elements according to their properties in a tabular
form.
All earlier attempts on the classification of elements were based on
atomic mass. Several chemists have for long tried to classify the elements and
to find patterns in their properties.
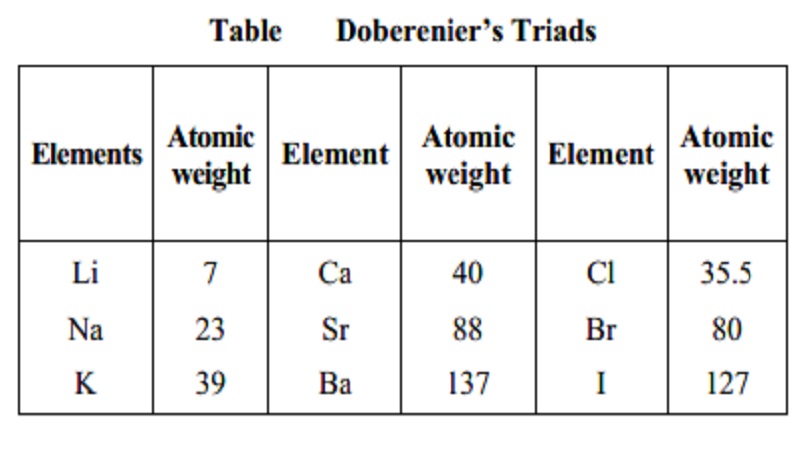
Dobereiner's Triads
In 1829, John Dobereiner (German Chemist)
classified elements having similar properties into groups of three. These
groups were called triads. According to this law when elements are arranged in
the order of increasing atomic mass in a triad, the atomic mass of the middle
element was found to be approximately equal to the arithmetic mean of the other
two elements. For example lithium, sodium and potassium constituted one triad.
However, only a limited number of elements could be grouped into traids.
Table Doberenier's Triads
Elements Atomic Element Atomic Element Atomic
weight weight weight
Li 7 Ca 40 Cl 35.5
Na 23 Sr 88 Br 80
K 39 Ba 137 I 127
Newlands Law of Octaves
In 1865, John Newlands (English Chemist) observed that if the elements
were arranged in order of their increasing atomic weights, the eighth element
starting from a given one, possessed properties similar to the first, like the
eighth note in an octave of music. He called it the law of octaves. It worked
well for the lighter elements but failed when applied to heavier elements.
Lother-Meyer's Arrangement
In 1869, J. Lother-Meyer in
Germany gave a more detailed and accurate relationship among the elements.,
Lother-Meyer plotted atomic volumes versus atomic weights of elements and
obtained a curve. He pointed out that elements occupying similar positions in
the curve possessed similar properties.
Mendeleev's Periodic Table
In 1869,
Dimitriv Mendeleev (Russian Chemist) arranged the 63 chemical elements, then
known, according to their increasing order of atomic weights. He gave his
famous scheme of the periodic classification of elements known as the periodic
law. The law states that ' the properties of the elements are the periodic
function of their atomic weights'. It means that when elements are arranged in
order of increasing atomic weights, the elements was similar properties recur
after regular intervals. On the basis of this periodic law Mendeleev
constructed a periodic table in such a way that the elements were arranged
horizontally in order of their increasing atomic weights. Mendeleev, while
studying his Periodic Table had found that in certain cases the regularity in
behaviour between two succeeding elements was not observed. In order to
overcome this he had kept gaps between such elements and had predicted that the
gaps would be filled by new elements, to be discovered in future, For example,
both gallium and germanium were not discovered at the time when Mendeleev
proposed the periodic table. Mendeleev named these elements as eka-aluminium
and eka-silicon because he believed that they would be similar to aluminium and
silicon respectively. These elements were discovered later and Mendeleev's
prediction proved remarkably correct. The discoveries / synthesis of new
elements have continued even to the present day, raising their number to 120.
The elements with atomic numbers upto 92 (i.e. uranium) are found in nature.
The rest known as transuranium elements have been synthesized in the
laboratories, which are highly unstable. They decay radioactively.
The modified periodic table is essentially similar to that of Mendeleev
with a separate column added for noble gases, which were not discovered until
the closing years of the nineteenth century. The general plan of the modified
Mendeleev's periodic table is improved.
The Mendeleev's modified periodic table consists of:
1.
Nine vertical columns called groups. These are
numbered from I to VIII and zero. (The members of zero group were not
discovered at the time of Mendeleev). Each group from I to VII is further
sub-divided into two sub-groups designated as A and B. Group VIII consists of
three sets, each one containing three elements. Group zero consists of inert
gases.
2.
Seven horizontal rows, called periods. These are
numbered from 1 to 7. First period contains two elements. Second and third
periods contain eight elements each. These periods are called short periods.
Fourth and fifth contains eighteen elements each. These periods are called long
periods. Sixth period contains thirty two elements and is called longest period.
Seventh period is incomplete and contains nineteen elements according to early
classification.
IUPAC periodic table
and IUPAC nomenclature of elements with atomic number greater than 100
Modern Periodic Law
In 1913, a British Physicist Henry Moseley showed that the atomic number
is a more fundamental property of an element than its atomic weight. This
observation led to the development of modern periodic law. The modern periodic
law states that ' the physical and chemical properties of the elements are
periodic function of their atomic numbers.'
This means that when the elements are arranged in order of increasing
atomic numbers, the elements with similar properties recur after regular
intervals. The periodic repetition is called periodicity. The physical and
chemical properties of the elements are related to the arrangement of electrons
in the outermost shell. Thus, if the arrangement of electrons in the outermost
shell (valence shell) of the atoms is the same, their properties will also be
similar. For example, the valence shell configurations of alkali metals show
the presence of one electron in the s-orbital of their valence shells.
Similar behaviour of alkali metals is attributed to the similar valence
shell configuration of their atoms. Similarly, if we examine the electronic
configurations of other elements, we will find that there is repetition of the
similar valence shell configuration after certain regular intervals with the
gradual increase of atomic number. Thus we find that the periodic repetition of
properties is due to the recurrence of similar valence shell configuration
after certain intervals. It is observed that similarity in properties is
repeated after the intervals of 2, 8, 18, or 32 in their atomic numbers.
Long form
of the Periodic Table: The periodic table is
constructed on the basis of
repeating electronic configurations of the atoms when they are arranged in the
order of increasing atomic numbers. The long form of the Periodic table is
given in a modified form in page number 70. Readers are advised to follow the
periodic table closely while studying the structural features of the long form
of the Periodic Table.
Structural
Features of the Long form of the periodic Table: The long form of the periodic
table consists of horizontal rows called periods and vertical columns called
groups.
Periods: In terms of electronic structure of the atom, a period constitutes a series of elements whose
atoms have the same number of electron shell i.e., principal quantum number
(n). There are seven periods and each period starts with a different principal
quantum number.
The first period corresponds to the filling of electrons in the first
energy shell (n = 1). Now this energy level has only one orbital (1s) and,
therefore, it can accommodate two electrons. This means that there can be only
two elements (hydrogen, 1s1 and helium, 1s2 ) in the
first period.
The second period starts with the electron beginning to enter the second
energy shell (n = 2). Since there are only four orbitals (one 2s-and three 2p-
orbitals) to be filled, it can accommodate eight electrons. Thus, second period
has eight elements in it. It starts with lithium (Z = 3) in which one electron
enters the 2s-orbital. The period ends with neon (Z = 10) in which the second
shell is complete (2s22p6).
The third period begins with the electrons
entering the third energy shell (n = 3). It should be noted that out of nine
orbitals of this energy level (one s, three p and five d) the five 3d-orbitals
have higher energy than 4s-orbitals. As such only four orbitals (one 3s and
three 3p) corresponding to n = 3 are filled before fourth energy level begins
to be filled. Hence, third period contains only eight elements from sodium (Z =
11) to argon (Z = 18).
The fourth period corresponding to n = 4 involves the filling of one 4s
and three 4p-orbitals (4d and 4f orbitals have higher energy than 5s-orbital
and are filled later). In between 4s and 4p-orbitals, five 3d-orbitals are also
filled which have energies in between these orbitals. Thus, altogether nine
orbitals (one 4s, five 3d and three 4p ) are to be filled and therefore, there
are eighteen elements in fourth period from potassium (Z = 19) to krypton (Z =
36). The elements from scandium (Z = 21) to zinc (Z = 30) are called 3d-
transition series.
The fifth period beginning with 5s-orbital (n=5) is similar to fourth
period. There are nine orbitals (one 5s, five 4d and three 5p) to be filled
and, therefore, there are eighteen elements in fifth period from rubidium (Z =
37) to xenon (Z = 54).
The sixth period starts with the filling of
6s-orbitals (n= 6). There are sixteen orbitals (one 6s, seven 4f, five 5d, and
three 6p) in which filling of electrons takes place before the next energy
level starts. As such there are thirty two elements in sixth period starting
from cesium (Z = 55) and ending with radon (Z = 86). The filling up of 4f
orbitals begins with cerium (Z = 58) and ends at lutetium (Z = 71). It
constitutes the first f-inner transition series which is called lanthanide
series.
The seventh period begins with 7s-orbital (n =
7). It would also have contained 32 elements corresponding to the filling of
sixteen orbitals (one 7s, seven 5f, five 6d and three 7p), but it is still
incomplete. At present there are 23 elements in it. The filling up of 5f-
orbitals begins with thorium (Z = 90) and ends up at lawrencium (Z = 103). It
constitutes second f-inner transition series which is called actinide series.
It mostly includes man made radioactive elements. In order to avoid undue
extension of the periodic table the 4f and 5f- inner transition elements are
placed separately.
The number of elements and the corresponding
orbitals being filled are given below.
Principal Orbitals Electrons
to Number of
Period Valence being filled be accommo-
shell
(=n) up dated electrons
First N
= 1 1s 2 2
Second N
= 2 2s, 2p 2+6 8
Third n
= 3 3s, 3p 2+6 8
Fourth n
= 4 4s, 3d, 4p 2 +10+6 18
Fifth n
= 5 5s, 4d, 5p 2+10+6 18
Sixth n
= 6 6s, 4f, 5d, 6p 2+14+10+6 32
Seventh n
= 7 7s, 5f, 6d, 7p 2+14+10+6 32
The first three periods containing 2, 8 and 8
elements respectively are called short periods, the next three periods
containing 18, 18 and 32 elements respectively are called long periods.
Groups
A vertical column in
the periodic table is known as group. A group consists of a series of elements having
similar configuration of the outer energy shell. There are eighteen vertical columns in long from of the periodic table.
According to the recommendations of the International
Union of Pure and Applied Chemistry (IUPAC),
these groups are numbered from 1 to
18. Previously, these were numbered from I to VII as A and B, VIII and zero
groups elements. The elements belonging to the same group are said to
constitute a family. For example, elements of group 17 (VII A) constitute
halogen family.
IUPAC Nomenclature for Elements with Z > 100
The elements beyond uranium (Z = 92) are all synthetic elements and are
known as transuranium elements. The elements beyond fermium are known as
transfermium elements. These elements fermium (Z = 100), mendelevium (Z = 101),
nobelium (Z = 102) and lawrencium (Z = 103) are named after the names of famous
scientists. Although names and symbols to many of these elements have been
assigned by these are still not universally accepted. Also some of these
elements have been assigned two names/symbols. For example, element with atomic
number 104 is called either Kurchatovium (Ku) or Rutherfordium (Rf) while
element with atomic number 107 is called Neilsbohrium (Ns) or Borium (Bh). But
the following elements have been assigned only one official name. For example
element with atomic number 105 is called Dubnium, with atomic number 106 as
Seaborgium, with atomic number 108 as Hassnium and with atomic number 109 is
named as Meiternium. To overcome all these difficulties, IUPAC nomenclature has
been recommended for all the elements with Z > 100. It was decided by IUPAC
that the names of elements beyond atomic number 100 should use Latin words for
their numbers. The names of these elements are derived from their numerical
roots.
Numerical -> 0 1 2 3 4 5 6 7 8 9
roots nil un bi tri quad pent hex sept oct en
Atomic Name
of the Symbol
number element
101 Unnilunnium Unu
102 Unnilbium Unb
103 Unniltrium Unt
104 Unnilquadium Unq
105 Unnilpentium Unp
106 Unnilhexium Unh
107 Unnilseptium Uns
108 Unniloctium Uno
109 Unnilennium Une
110 Ununnilium Uun
111 Unununium Uuu
112 Ununbium Uub
113 Ununtrium Uut
114 Ununquadium Uuq
115 Ununpentium Uup
116 Ununhexium Uuh
117 Ununseptium Uus
118 Ununoctium Uuo
119 Ununennium Uue
120 Unbinilium Ubn
Related Topics