Chapter: Medical Physiology: Regulation of Acid-Base Balance
Bicarbonate Buffer System
Bicarbonate Buffer System
The bicarbonate buffer system consists of a water solu-tion that contains two ingredients: (1) a weak acid, H2CO3, and (2) a bicarbonate salt, such as NaHCO3.
H2CO3 is formed in the body by the reaction of CO2 with H2O.
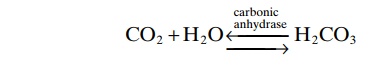
This reaction is slow, and exceedingly small amounts of H2CO3 are formed unless the enzyme carbonicanhydrase is present. This enzyme is especially abun-dant in the walls of the lung alveoli, where CO2 is released; carbonic anhydrase is also present in the epithelial cells of the renal tubules, where CO2 reacts with H2O to form H2CO3.
H2CO3 ionizes weakly to form small amounts of H+ and HCO3–.
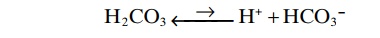
The second component of the system, bicarbonate salt, occurs predominantly as sodium bicarbonate (NaHCO3) in the extracellular fluid. NaHCO3 ionizes almost completely to form HCO3– and Na+, as follows:
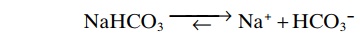
Now, putting the entire system together, we have the following:
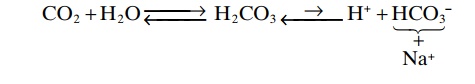
Because of the weak dissociation of H2CO3, the H+ concentration is extremely small.
When a strong acid such as HCl is added to the bicarbonate buffer solution, the increased H+ released from the acid (HCl - - > H+ + Cl–) is buffered by HCO3–.
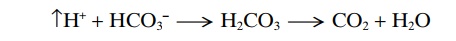
As a result, more H2CO3 is formed, causing increased CO2 and H2O production. From these reactions, one can see that H+ from the strong acid HCl reacts with HCO3– to form the very weak acid H2CO3, which in turn forms CO2 and H2O. The excess CO2 greatly stim-ulates respiration, which eliminates the CO2 from the extracellular fluid.
The opposite reactions take place when a strong base, such as sodium hydroxide (NaOH), is added to the bicarbonate buffer solution.
NaOH + H2CO3 --- > NaHCO3 + H2O
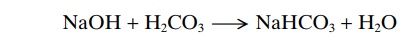
In this case, the OH– from the NaOH combines with H2CO3 to form additional HCO3–. Thus, the weak base NaHCO3replaces the strong base NaOH. At the same time, the concentration of H2CO3 decreases (because it reacts with NaOH), causing more CO2 to combine with H2O to replace the H2CO3.
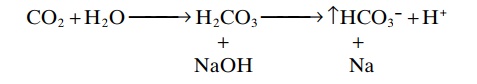
The net result, therefore, is a tendency for the CO2 levels in the blood to decrease, but the decreased CO2 in the blood inhibits respiration and decreases the rate of CO2 expiration. The rise in blood HCO3– that occurs is compensated for by increased renal excretion of HCO3–.
Related Topics