Chapter: Essential Microbiology: Biochemical Principles
Atomic structure
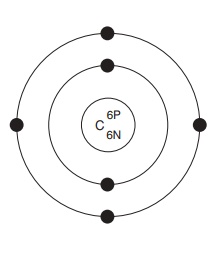
Atomic structure
All atoms have a central, positively charged nucleus, which is very dense, and makes up most of the mass of the atom. The nucleus is made up of two types of particle, protons and neutrons. Protons carry a positive charge, and neutrons are uncharged,hence the nucleus overall is positively charged. It is surrounded by much lighter, and rapidly orbiting, electrons (Figure 2.1). These are negatively charged, the charge being equal (but of course opposite) to that of the protons, but they have only 1/1840 of the mass of either protons or neutrons. The attractive force between the positively charged protons and the negatively charged electrons holds the atom together.
The number of protons in the nucleus is called the atomic number, and ranges from 1 to over 100. The combined total of protons and neutrons is known as the mass number. All atoms have an equal number of protons and electrons, so regardless of the atomic number, the overall charge on the atom will always be zero.
Atoms having the same atomic number have the same chemical properties; such atoms all belong to the same element. An element is made up of one type of atom only and cannot be chemically broken down into simpler substances; thus pure copper for example is made up entirely of copper atoms. There are 92 of these elements occurring naturally, 26 of which commonly occur in living things. Each element has been given a universally agreed symbol; examples which we shall encounter in biological macro-molecules include carbon (C), hydrogen (H) and oxygen (O). The atomic numbers of selected elements are shown in Table 2.2.
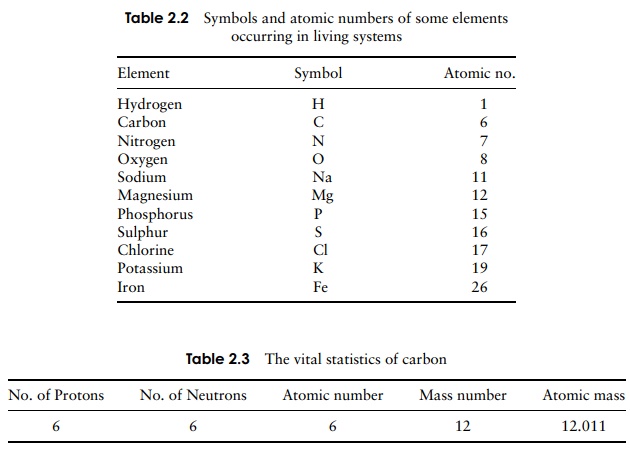
The relationship between neutrons, protons, atomic number, and mass number is illustrated in Table 2.3, using carbon as an example, since all living matter is based upon this element. The carbon represented can be expressed in the form:
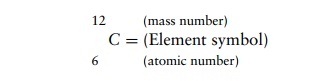
The number of neutrons in an atom can be deduced by subtracting the atomic number from the mass number. In the case of carbon, this is the same as the number of protons (6), but this is not always so. Phosphorus for example has 15 protons and 16 neutrons, giving it an atomic number of 15 and a mass number of 31.
Isotopes
Although the number of protons in the nucleus of a given element is always the same, the number of neutrons can vary, to give different forms or isotopes of that element. Carbon-14 (14C) is a naturally occurring but rare isotope of carbon that has eight neu-trons instead of six, hence the atomic mass of 14. Carbon-13 (13C) is a rather more com-mon isotope, making up around 1 per cent of naturally occurring carbon; it has seven neutrons per atomic nucleus. The atomic mass (or atomic weight) of an element is the average of the mass numbers of an element’s different isotopes, taking into account the proportions in which they occur. Carbon-12 is by far the predominant form of the element in nature, but the existence of small amounts of the other forms means that the atomic mass is 12.011. Some isotopes are stable, while others decay spon-taneously, with the release of subatomic particles. The latter are called radioisotopes; 14C is a radioisotope, while the other two forms of carbon are stable isotopes. Radioisotopes have been an extremely useful research tool in a number of areas of molecular biology.
The electrons that orbit around the nucleus do not do so randomly, but are arranged in a series of electron shells, radiating out from the nucleus (Figure 2.1). These layers correspond to different energy levels, with the highest energy levels being located furthest away from the nucleus. Each shell can accommodate a maximum number of electrons, and electrons always fill up the shells starting at the innermost one, that is, the one with the lowest energy level. In our example, carbon has filled the first shell with two electrons, and occupied four of the eight available spaces on the second.
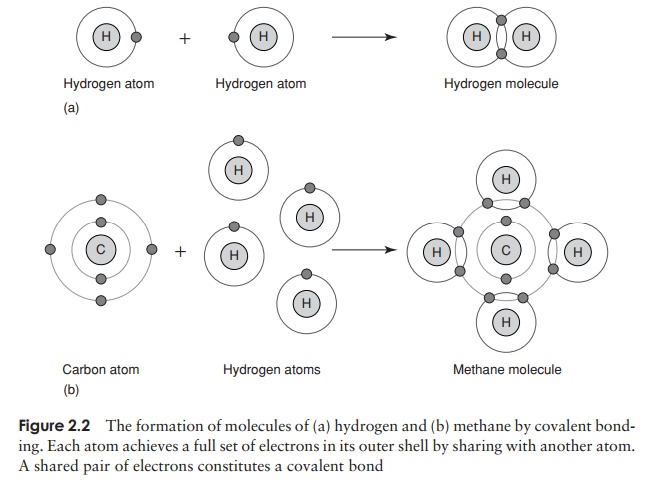
The chemical properties of atoms are determined by the number of electrons in the outermost occupied shell. Neon, one of the ‘noble’ gases, has an atomic number of 10, completely filling the first two shells, and is chemically unreactive or inert. Atoms that do not achieve a similar configuration are unstable, or reactive. Reactions take place between atoms that attempt to achieve stability by attaining a full outer shell. These reactions may involve atoms of the same element or ones of different elements; the result in either case is a molecule or ion . Figure 2.2 shows how atoms combine to form a molecule. A substance made up of molecules containing two or more different elements is called a compound. In each example, the product of the reaction has a full outer electron shell; note that some atoms are donating electrons, while others are accepting them.
The number of unfilled spaces in the outermost electron shell determines the reactivity of an atom. If most of the spaces in the outermost shell are full, or if most are empty, atoms tend to strive for stability by gaining or losing electrons, as shown in Figure 2.3.
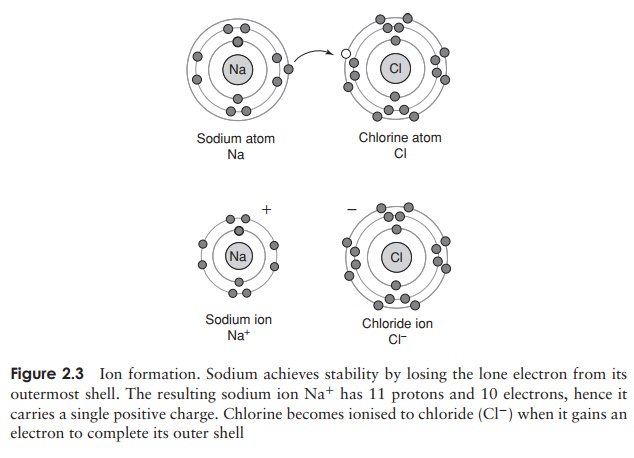
When this happens, an ion is formed, which carries either a positive or negative charge. Positively charged ions are called cations and negatively charged ones anions. The sodium atom for example has 11 electrons, meaning that the inner two electron shells are filled and a lone electron occupies the third shell. If it were to lose this last electron, it would have more protons thanelectrons, and therefore have a net positive charge of one; if this happened, it would become a sodium ion, Na+ (Figure 2.3).
Chemical bonds
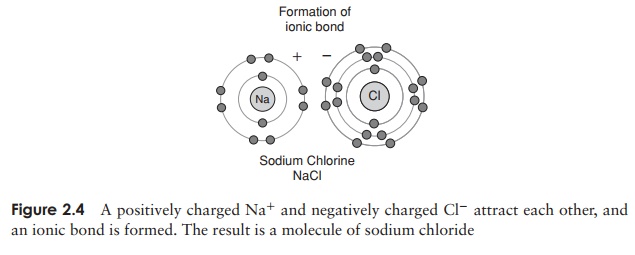
The force that causes two or more atoms to join together is known as a chemical bond, and several types are found in biological systems. The interaction between sodium and chloride ions shown in Figure 2.4 is an example of ionic bonding, where the transfer of an electron from one party to another means that both achieve a complete outer electron shell. There is an attractive force between positively and negatively charged ions, called an ionic bond. Certain elements form ions with more than a single charge, by gaining or losing two or more electrons in order to achieve a full outer electron shell; thus calcium ions (Ca2+ ) are formed by the loss of two electrons from a calcium atom.
The goal of stability through a full complement of outer shell electrons may also be achieved by means of sharing one or more pairs of electrons. Consider the formation of water (Figure 2.2); an oxygen atom, which has two spaces in its outer shell, can achieve a full complement by sharing electrons from two separate hydrogen atoms. This type of bond is a covalent bond.
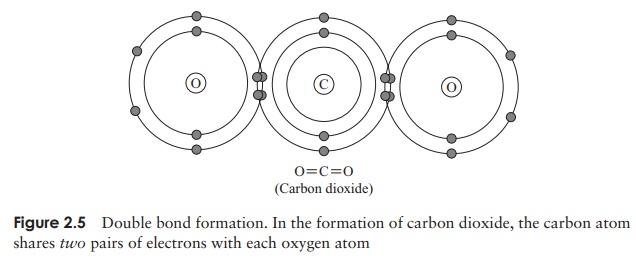
Sometimes, a pair of atoms share not one but two pairs of electrons (see Figure 2.5). This involves the formation of a double bond. Triple bonding, through the sharing of three pairs of electrons, is also possible, but rare.
In the examples of covalent bonding we’ve looked at so far, the sharing of the electrons has been equal, but this is not always the case because sometimes the electrons may be drawn closer to one atom than another (Figure 2.6a). This has the effect of making one atom slightly negative and another slightly positive. Molecules like this are called polar molecules and the bonds are polar bonds. Sometimes a large molecule may have both polar and non-polar areas.
Polar molecules are attracted to each other, the negative areas of one molecule and the positive areas of another acting as magnets for one another (Figure 2.6b). In water,
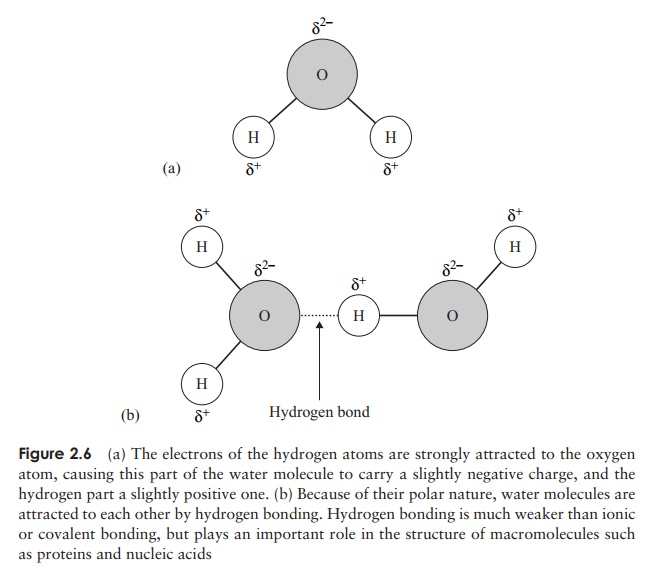
bonds; however, if sufficient of them form in a compound the overall bonding force can be appreciable. Each water molecule can form hydrogen bonds with others of its kind in four places (Figure 2.6b). In order to break all these bonds, a large input of energy is required, explaining why water has such a relatively high boiling point, and why most of the water on the planet is in liquid form.
Another weak form of interaction is brought about by Van der Waals forces, which occur briefly when two non-polar molecules (or parts of molecules) come into very close contact with one another. Although transient, and generally even weaker than hydrogen bonds, they occur in great numbers in certain macromolecules and play an important role in holding proteins together .
Water is essential for living things, both in the composition of their cells and in the environment surrounding them. Organisms are made up of between 60 and 95 per cent water by weight, and even inert, dormant forms like spores and seeds have a significant water component. This dependence on water is a function of its unique properties, which in turn derive from its polar nature.
Water is the medium in which most biochemical reactions take place; it is a highly efficient solvent, indeed more substances will dissolve in water than in any other sol-vent. Substances held together by ionic bonds tend to dissociate into anions and cations in water, because as individual solute molecules become surrounded by molecules of water, hydration shells are formed, in which the negatively charged parts of the solute attract the positive region of the water molecule, and the positive parts the negative region (Figure 2.7). The attractive forces that allow the solute to dissolve are called hy-drophilic forces, and substances which are water-soluble are hydrophilic (water-loving).Other polar substances such as sugars and proteins are also soluble in water by forming hydrophilic interactions.
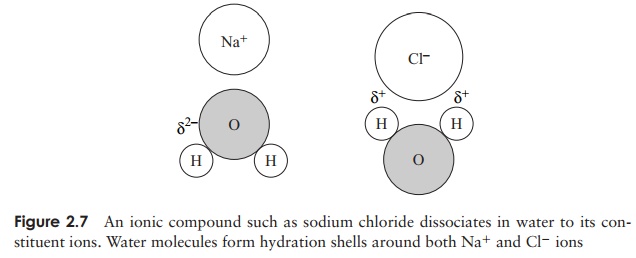
ions. Water molecules form hydration shells around both Na+ and Cl− ions Molecules such as oils and fats are non-polar, and because of their non-reactivity with water are termed hydrophobic (‘water-fearing’). If such a molecule is mixed with water, it will be excluded, as water molecules ‘stick together’. This very exclusion by water can act as a cohesive force among hydrophobic molecules (or hydrophobic areas of large molecules). This is often called hydrophobic bonding, but is not really bonding as such, rather a shared avoidance of water. All living cells have a hydrophilic interior surrounded by a hydrophobic membrane.
An amphipathic substance is one which is part polar and part non-polar. When such a substance is mixed with water, micelles are formed (Figure 2.8); the non-polar
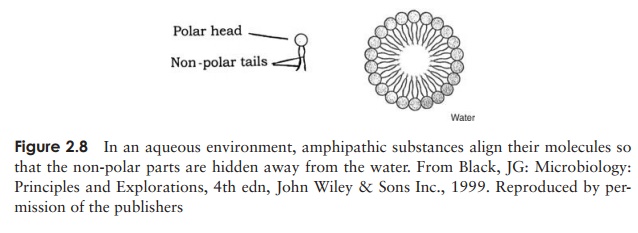
parts are excluded by the water and group together as described above, leaving the polar groups pointing outwards into the water, where they are attracted by hydrophilic forces. Detergents exert their action by trapping insoluble grease inside the centre of a micelle, while interaction with water allows them to be rinsed away.
Water takes part in many essential metabolic reactions, and its polar nature allows for the breakdown to hydrogen and hydroxyl ions (H+ and OH− ), and re-synthesis as water. Water acts as a reactant in hydrolysis reactions such as:
A--B + H2O → A--H + B--OH
and as a product in certain synthetic reactions, such as:
A--H + B--OH → A--B + H2O
Related Topics