Chapter: Pharmaceutical Drug Analysis: Atomic Absorption Spectroscopy
Atomic Absorption Spectroscopy: Instrumentation
INSTRUMENTATION
The atomic absorption spectrophotometers are essentially
of two types, namely :
(a) Single-beam
Atomic Absorption Spectrophotometer, and
(b) Double-beam
Atomic Absorption Spectrophotometer.
These two instruments shall be discussed briefly here
along with their vital components.
1. SINGLE-BEAM ATOMIC ABSORPTION SPECTROPHOTOMETER
The schematic diagram of a single-beam atomic
spectrophotometer in illustrated in Figure 26.1.
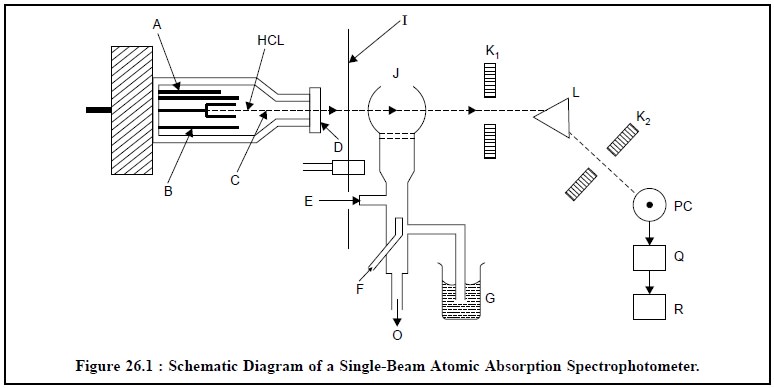
A = Anode (Tungsten),
B= Glass-shield,
C= Neon or Argon at 1-5 torr,
D= Quartz or Pyrex Window,
E= Inlet for Acetylene,
F = Inlet for air,
G = Liquid sample sucked in by an atomizer,
HCL = Hollow cathode Lamp,
I = Chopper (a rotating shutter),
J = Flame,
K1 and K2 = Slits,
L = Prism or Grating,
M = Photocell,
O = Drain outlet to maintain a constant pressure head in
the mixing chamber,
PC = Photocell,
Q = Photodetector, and
R = Amplifier and Recorder.
The most common source for atomic absorption measurements
in the ‘hollow-cathode-lamp’, which essentially consists of a Tungsten anode
(A) and a cylindrical cathode (HCL) sealed in a glass tube (B) that is duly
filled with neon or Argon (C) at a pressure of 1 to 5 torr. It is a practice to
have the cathode constructed of the metal whose spectrum is desired or serves
to support a layer of that particular metal. The chopper* (I) is interposed
between the hollow-cathode-lamp (HCL) and the flame (J). Subsequently, the
liquid sample (G) is sucked in by an atomizer into the flame (J). Just prior to
its entry to the flame, the sample solution first gets dispersed into a mist of
very small droplets that evaporates in the flame to yield initially the dry
salt, and subsequently the vapour of the salt. At this particular stage a
portion of this vapour will be dissociated into atoms of the element required
to be measured. In this manner, the flame possesses free ground state (i.e., unexcited) atoms that are worthy
of absorbing radiations, from an external source when the radiation eventu-ally
matches exactly to the energy needed for a transition element from the lower
ground-state-level to the upper excited-state-level. The resulting unabsorbed
radiation from the flame (J), firstly passes through the slit (K1)
and then through the monochromator i.e.,
the prism or grating (L) that exclusively isolates the exciting spectral lines
of the light source ; secondly, through the slit (K2) into the
photocell (PC), thirdly, into a photodetector (Q) and fourthly, its output is
adequately amplified and registered on a recorder (R). It is worthwhile to
mention here that the final absorption is measured by the difference in the
transmitted signal both in the absence and presence of the element under
investigation.
2. DOUBLE-BEAM ATOMIC ABSORPTION SPECTROPHOTOMETER
The major disadvantage of a single-beam atomic absorption
spectrophotometer (Figure 26.1) lies in its very low stability. The
introduction of a double-beam atomic
absorption spectrophotometer (Figure 26.2) has completely eliminated the
above main lacuna and provides much enhanced stability. In this particu-lar
instance the chopped beam of light from the hollow-cathode-lamp is split into
two parts. The first portion, passes through the flame, while the second
portion is made to bypass the flame completely. However, the two separate beams
of light are recombined meticulously by an unique optically-designed assembly,
passes through a monochromator to a strategically placed detector and
ultimately to a sensitive read-out device.
It is pertinent to mention here that a double-beam atomic
absorption spectrophotometer is absolutely independent of (a) lamp drift, (b)
sensitivity of detector with time.
The optical path of a double-beam atomic absorption
spectrophotometer is depicted in Figure 26.2. The various essential components
comprising the optical arrangement in Figure 26.2 are enumerated after the
figure.
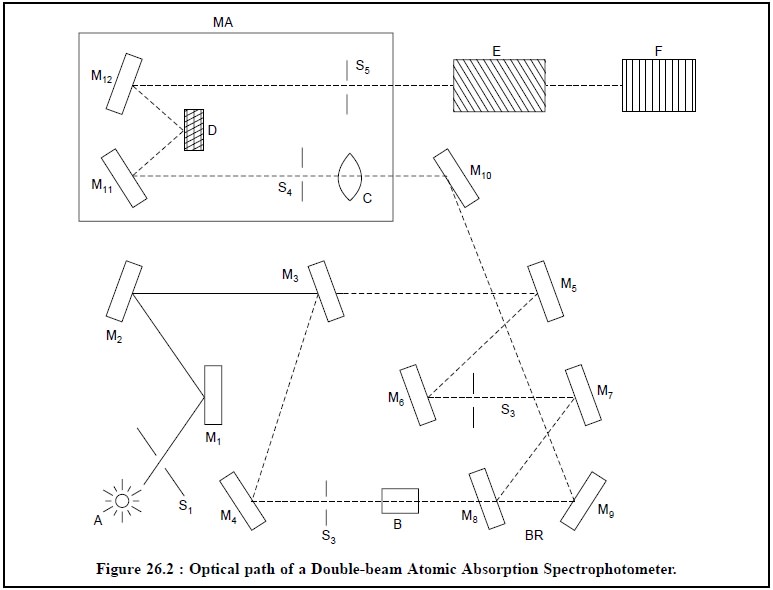
A = Source of light (Hollow-Cathode-Lamp),
B = Flame,
C = Field lens,
D = Grating,
E = Detector,
F = Read-out device,
BR = Beam recombination zone,
MA = Monochromator assembly,
S1 to S4 = Slits,
S5 = Exit slit, and
M1 to M12 = Mirrors
The light hollow-cathode-lamp source (A) passes through
the slit S1 and strikes at mirrors M1 and M2.
The Mirrors M3 splits chopped beam from the source into two parts ;
one passes through the mirror M4-slit S2-flame (B)-mirror
M8 and strikes at mirror M9 to reach mirror M10,
and the second strikes at mirror M6-slit S3-mirror M7,
M8 and M9 respectively to reach the mirror M10.
The mirror M8 and M9 serve as a beam recombination zone (BR).
The recombined beam gets reflected by mirrors M10 passes through the field lens (C), slit S4, strikes at M11,
passes through the grating (D), to the mirror M12 and ultimately
passes out through the exit (S5) and the monochromator assembly (MA)
into the detector (E) and finally to the read-out device (F).
Related Topics