Nomenclature and Isomerism, Aromaticity, Structure, Sources | Chemistry - Aromatic Hydrocarbons | 11th Chemistry : UNIT 13 : Hydrocarbons
Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Aromatic Hydrocarbons
Aromatic
Hydrocarbons
Take
a moment and think of sub-stances that have a strong fragrance. What kind of
things come to your mind?
Perfume,
Vanila or cinnamon? They smell differently, they have something in common.
These substances are made of aromatic compounds [Greek: Aroma-Pleasant
smelling]. However, some compounds are chemically aromatic but do not have
distinct smell. The aromatic hydrocarbons are classified depending upon number
of rings present in it.
(i)
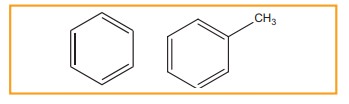
Monocyclic aromatic hydrocarbon (MAH)
(Ex)
Benzene (C6H6) and Toluene (C7H8)
(ii)
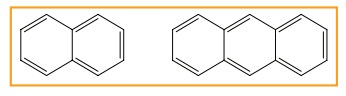
Polycyclic aromatic hydrocarbon(PAH)
(Ex)
Naphthalene (C10H8) and Anthra-cene (C14H10)
Nomenclature and Isomerism
•
We have already discussed about nomenclature of aromatic hydrocarbons in
Unit:11. The first member of aromatic hydrocarbon is benzene (C6H6)
represented by a regular hexagon with a circle inscribed in it.
•
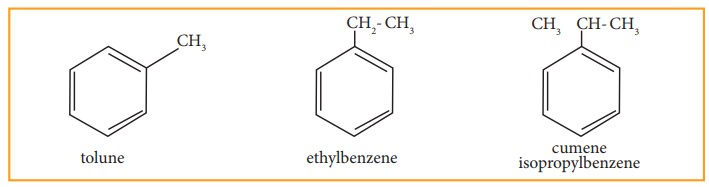
Since, all the six hydrogen atom in benzene are equivalent, it can give only
one mono-substituted compound (Ex) methyl benzene (C6H5-CH3)
which named as toluene.
•
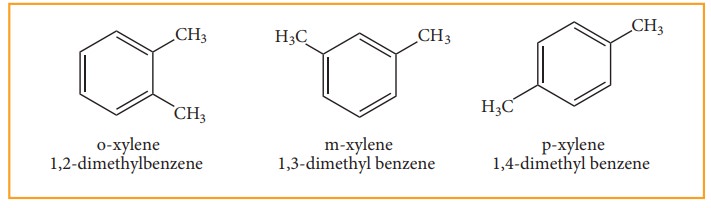
When di substitution occurs either by a similar monovalent atom or two
different atoms or groups in benzene, then three different position isomers are
possible. Their relative positions are indicated as ortho (1,2), meta (1,3) and
para (1,4). For example, consider dimethyl benzene which is named as xylene.
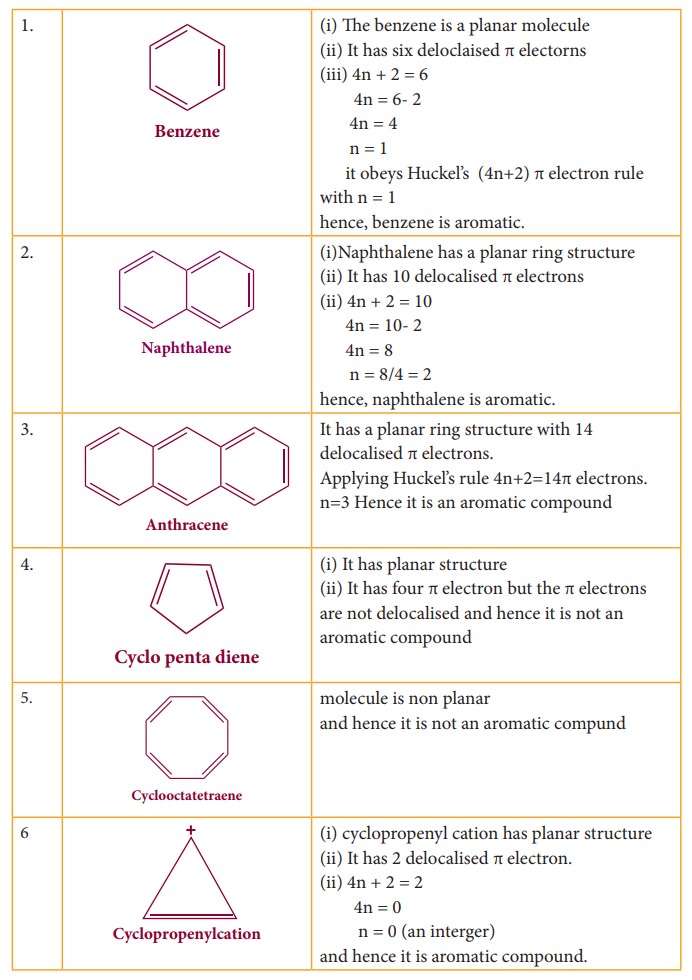
Aromaticity
Huckel
proposed that aromaticity is a function of electronic structure. A compound may
be aromatic, if it obeys the following rules
i.
The molecule must be co-planar
ii.
Complete delocalization of π electron in the ring
iii.
Presence of (4n+2) π electrons in the ring where n is an integer (n=0,1,2….)
This
is known as Huckel’s rule.
Some
of the examples for Huckel rule
Structure of benzene:
1. Molecular formula:v
Elemental
Analysis and molecular weight determination have proved that the molecular
formula of benzene is C6H6. This indicates that benzene
is a highly unsaturated compound.
2. Straight chain structure not possible:
Benzene
could be constructed as a straight chain or ring compound but it not feasible
since it does not show the properties of alkenes or alkynes.for example, it did
not decolourise bromine in carbon tetrachloride or acidified KMnO4.
It did not react with water in the presence of acid.
3. Evidence of cyclic structure:
i) substitution of benzene:
Benzene
reacts with bromine in the presence of AlCl3 to form mono
bromobenzene.
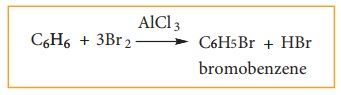
Formation
of only one monobromo compound indicates that all the six hydrogen atoms in
benzene were identical. This is possible only if it has a cyclic structure of
six carbons each containing one hydrogen.
ii) addition of hydrogen:
Benzene
can add on to three moles of hydrogen in the presence of nickel catalyst to
give cyclohexane.
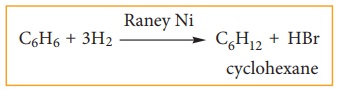
This confirms cyclic structure of ben-zene and the presence of three carbon-car-bon double bond.
4. Kekule’s structure of benzene:
In
1865, August Kekule suggest-ed that benzene consists of a cyclic planar
structure of six carbon with alternate sin-gle and double bonds.
There
were two objections:
i)
Benzene forms only one orthodisub-stituted products whereas the Kekule’s
structure predicts two o-di substituted products as shown below.
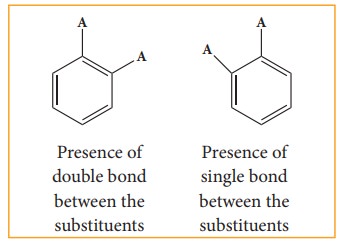
ii)
Kekule’s structure failed to explain why benzene with three double bonds did
not give addition reactions like other alkenes.To overcome this objection,
Kekule suggested that benzene was mixture of two forms (1 and 2)which are in
rapid equilibrium.
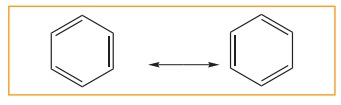
5. Resonance description of benzene:
The
phenomenon in which two or more structures can be written for a substance which
has identical position of atoms is called resonance. The actual structure of
the molecule is said to be resonance hybrid of various possible alternative
structures. In benzene, Kekule’s structures I & II represented the
resonance structure, and structure III is the resonance hybrid of structure I
&II
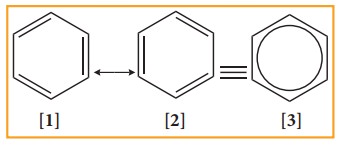
The
structures 1 and 2 exist only in theory. The actual structure of benzene is the
hybrid of two hypothetical resonance structures.
6. Spectrosscopic measurments:
Spectroscopic
measurements show that benzene is planar and all of its car-bon-carbon bonds
are of equal length 1.40A°. This value lies between carbon-car-bon single bond
length 1.54A° and car-bon-carbon double bond length 1.34A°.
7. Molecular orbital structre:
The
structure of benzene is best de-scribed in terms of the molecular orbital
theory. All the six carbon atoms of benzene are sp2 hybridized. Six
sp2 hybrid orbitals of carbon leanerly overlap with six one is
or-bitals of hydrogen atoms to form six C - H sigma bonds. Overlap between the
remain-ing sp2 hybrid orbitals of carbon forms six C-C sigma bonds.
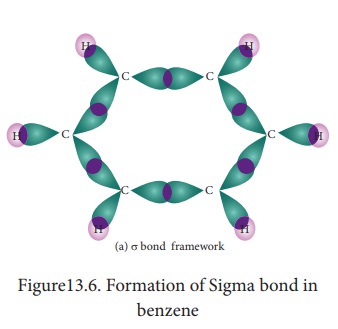
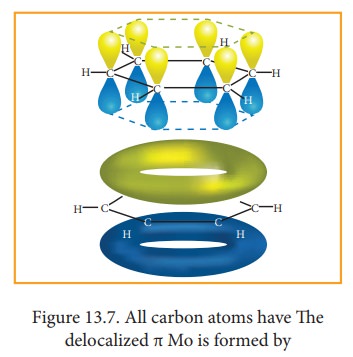
All
the σ bonds in benzene lie in one plane with bond angle 120°. Each carbon atom
in benzene possess an un hybridized p-orbital containing one electron. The
lateral overlap of their p-orbital produces 3 π- bond The six electrons of the
p-orbitals cover all the six carbon atoms and are said to be delocalised. Due
to delocalization, strong π-bond is formed which makes the molecule stable.
Hence unlike alkenes and alkynes benzene undergoes substitution reactions
rather addition reactions under normal conditions.
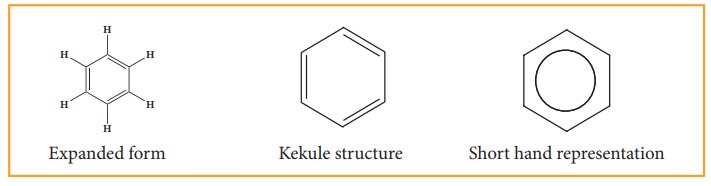
8. Representation of benzene:
Hence,
there are three ways in which benzene can be represented.
Benzene and its homologous series
Benzene
and its homologous series are colorless liquids with pleasant odour .They are
lighter than water and insoluble in it. Their vapours are highly flammable, and
volatile and toxic in nature.
Sources of aromatic compound:
Benzene
and other aromatic compound are obtained from coal tar and petroleum
It
can also be prepared in laboratory using some simple aliphatic compounds
1. Preparation of benzene
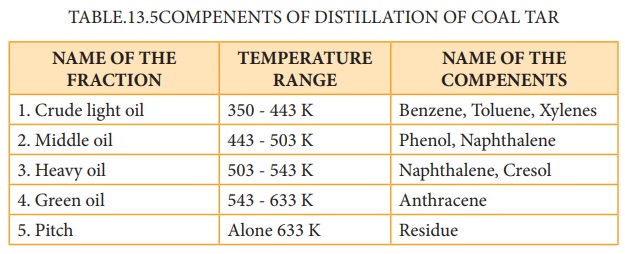
(i) industrial preparation of benzene from coal tar :
Coal
tar is a viscous liquid obtained by the pyrolysis of coal. During fractional
distillation, coal tar is heated and distills away its volatile compounds
namely benzene, toluene, xylene in the temperature range of 350 to 443 K. These
vapours are collected at the upper part of the fractionating column (Table
13.5.)
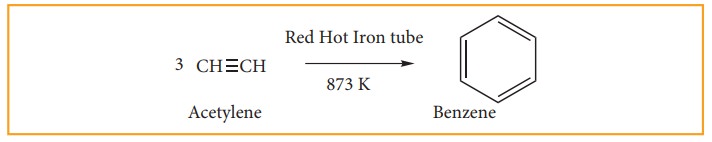
(ii) from acetylene.
Acetylene
on passing through a red –hot tube trimerises to give benzene. We have already
studied this concept in polymerization of alkynes.
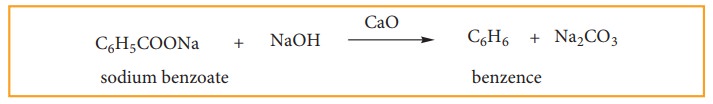
(iii) Laboratory Methods Of Preparing Benzene And Toluene
(a) Decarboxylaation Of Aromatic Acid.
When
sodium benzoate in heated with sodalime, benzene vapours distil over.
(b) Preparation Of Benzene From Phenol
When
phenol vapours are passed over zinc dust, then it is reduced to benzene.

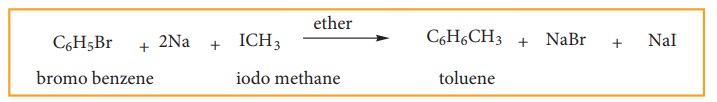
(c) Wurtz – Fittig Reaction:
When
a solution of bromo benzene and iodo methane in dry ether is treated with metallic
sodium, toluene is formed.
(d) Friedel Craft’s Reaction:
When
benzene is treated with methyl chloride in the presence of anhydrous aluminium
chloride, toluene is formed.

Related Topics