Chapter: 11th Chemistry : UNIT 3 : Periodic Classification of Elements
Anomalies of MendeleevŌĆÖs Periodic Table
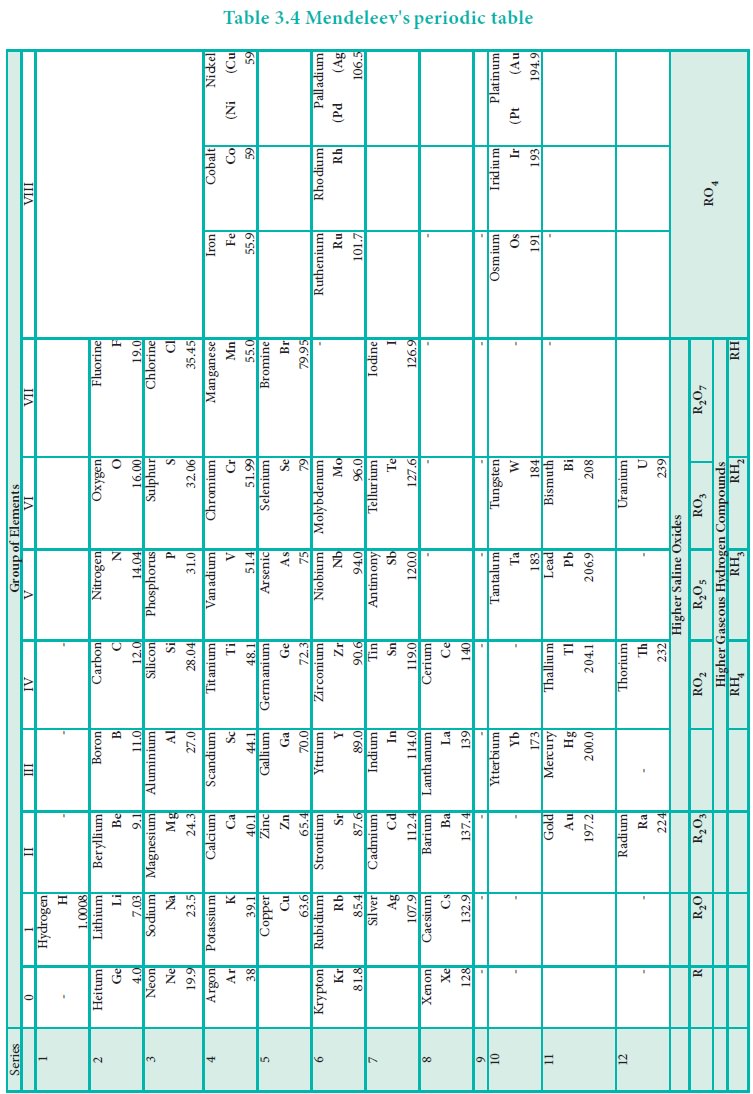
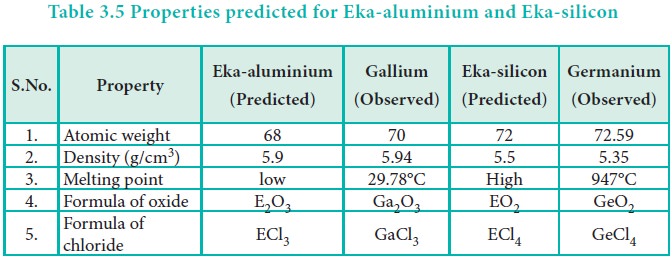
Some elements with similar properties were placed in different groups and those with dissimilar properties were placed in same group.
Anomalies
of MendeleevŌĆÖs Periodic Table
Some elements with similar properties were placed in
different groups and those with dissimilar properties were placed in same
group.
Example: Tellurium (127.6) was placed in VI group but
Iodine (127.0) was placed in VII group.
Similarly elements with higher atomic weights were placed
before lower atomic weights based on their properties in contradiction to his
periodic law. Example 59Co27 was placed before 58.7Ni28
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
11th Chemistry : UNIT 3 : Periodic Classification of Elements : Anomalies of MendeleevŌĆÖs Periodic Table |
Related Topics
11th Chemistry : UNIT 3 : Periodic Classification of Elements