Chapter: Clinical Anesthesiology: Anesthetic Management: Obstetric Anesthesia
Anesthesia for Neonatal Resuscitation
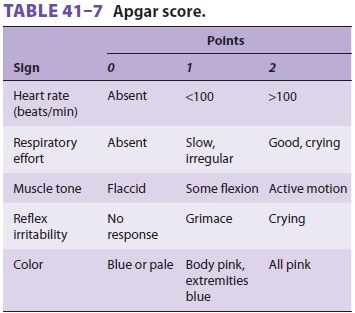
NEONATAL RESUSCITATION
1. General Care of the Neonate
One healthcare provider whose sole
responsibility is to care for the neonate and who is capable of provid-ing
resuscitation should attend every delivery. As the head is delivered, the nose,
mouth, and pharynx are suctioned with a bulb syringe. After the remainder of
the body is delivered, the skin is dried with a ster-ile towel. Once the umbilical
cord stops pulsating or neonatal breathing is initiated, the cord is clamped
and the neonate is placed in a radiant warmer with the bed tilted in a slight
Trendelenburg position.
Neonatal evaluation and treatment are carried
out simultaneously (Figure 41–5).
If the neonate is obviously depressed, the cord is clamped early and
resuscitation is initiated immediately. Breathing normally begins within 30 s
and is sustained within 90 s. Respirations should be 30–60 breaths/min and the
heart rate 120–160 beats/min. Respirations are assessed by auscultation of the
chest, whereas heart rate is determined by palpation of the pulse at the base
of the umbilical cord or auscultation of the pre-cordium. It is critically
important to keep the neo-nate warm.
In addition to respirations and heart rate, color, tone, and reflex
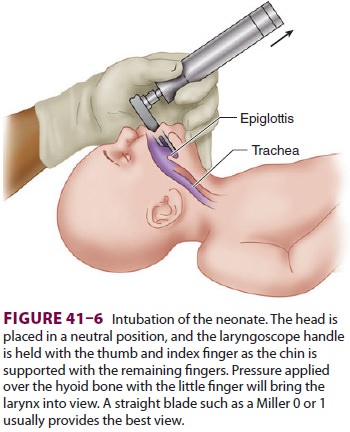
irritability should be evalu-ated. The Apgar score (Table 41–7),
recorded at 1 min and again at 5 min after delivery, remains the most valuable
assessment of the neonate. The 1-min score correlates with survival, whereas
the 5-min score has limited relationship to neurologi-cal outcome.
Neonates with Apgar scores of 8–10 are vig-orous and may require only
gentle stimulation (flicking the foot, rubbing the back, and additional
drying). A catheter should first be gently passed
through each nostril to rule out choanal atresia, and then through the
mouth to suction the stomach and rule out esophageal atresia.
2. Meconium-Stained Neonates
The presence or absence of meconium in the
amni-otic fluid (approximately 10–12% of deliveries) changes the immediate
management of the neonate at birth. Fetal distress, particularly after 42 weeks
of gestation, is often associated with release of thick meconium into the
fluid. Fetal gasping during stress results in entry of a large amount of
meconium-tainted amniotic fluid into the lungs. When the neonate initiates
respiration at birth, the meconium moves from the trachea and large airways
downtoward the periphery of the lung. Thick or partic-ulate meconium may
obstruct small airways and cause severe respiratory distress in 15% of
meco-nium-stained neonates. Moreover, these infants can develop persistent
fetal circulation.
Unless the neonate has absent or depressed respirations, thin watery
meconium does not require suctioning beyond careful bulb suctioning of the
oropharynx when the head emerges from the perineum (or from the uterus at
cesarean section). When thick “pea soup” meconium is present in the amniotic
fluid, however, some clinicians intubate and suction the trachea immediately
after delivery but before the first breath is taken. If the baby is not
vigorous, tracheal suctioning is recommended when meconium is present. Tracheal
suctioning of the thick meconium is accomplished by a special suctioning device
attached to the endotracheal tube as the tube is withdrawn. If meconium is
aspirated from the trachea, the procedure should be repeated until no meconium
is obtained—but no more than three times, after which it is usually of no
further benefit. The infant should then be given supplemen-tal oxygen by face
mask and observed closely. The stomach should also be suctioned to prevent
pas-sive regurgitation of any meconium. Newborns with meconium aspiration have
an increased incidence of pneumothorax (10% compared with 1% for all vagi-nal
deliveries).
3. Care of the Depressed Neonate
Approximately 6% of newborns, most of whom
weigh less than 1500 g, require some form of advanced life support.
Resuscitation of the depressed neonate requires two or more persons— one to
manage the airway and ventilation and another to perform chest compressions, if
neces-sary. A third person greatly facilitates the placement of intravascular
catheters and the administration of fluids or drugs. The anesthesiologist
caring for the mother can render only brief assistance and only when it does
not jeopardize the mother; other per-sonnel are, therefore, usually responsible
for neona-tal resuscitation.
Because the most common cause of neonatal depression is intrauterine
asphyxia, the empha-sis in resuscitation is on respiration. Hypovolemia is also
a contributing factor in a significant num-ber of neonates. Factors associated
with hypovole-mia include early clamping of the umbilical cord, holding the
neonate above the introitus prior to clamping, prematurity, maternal
hemorrhage, pla-cental transection during cesarean section, sepsis, and
twin-to-twin transfusion.
Failure of the neonate to quickly respond to respiratory resuscitative
efforts mandates vascular access and blood gas analysis; pneumothorax (1%
incidence) and congenital anomalies of the airway, including tracheoesophageal
fistula (1:3000–5000 live births), and congenital diaphragmatic hernia (1:2000–4000)
should also be considered.
Grouping by the 1-min Apgar score greatly
facilitates resuscitation: (1) mildly asphyxiated neo-nates (Apgar score of
5–7) usually need only stimu-lation while 100% oxygen is blown across the
face;moderately asphyxiated neonates (Apgar score of 3–4) require temporary
assisted positive-pressure ventilation with mask and bag; and (3) severely
depressed neonates (Apgar score of 0–2) should be immediately intubated, and
chest compressions may be required.
Guidelines for Ventilation
Indications for positive-pressure ventilation include apnea, (2) gasping respirations, (3) persistent central cyanosis with 100% oxygen, and (4) a per-sistent heart rate less than 100 beats/min. Excessive flexion or extension of the neck can cause airway obstruction. A 1-in.-high towel under the shoulders may be helpful in maintaining proper head posi-tion. Assisted ventilation by bag and mask should be at a rate of 30–60 breaths/min with 100% oxy-gen. Initial breaths may require peak pressures of up to 40 cm H2O, but pressures should not exceed 30 cm H2O thereafter. Adequacy of ventilation should be checked by auscultation and chest excur-sions. Gastric decompression with an 8F tube often facilitates ventilation. If after 30 s the heart rate is over 100 beats/min and spontaneous ven-tilations become adequate, assisted ventilation is no longer necessary. If the heart rate remains less than 60 beats/min or 60–80 beats/min without an increase in response to resuscitation, the neonate is intubated and chest compressions are started.
If the heart rate is 60–80 beats/min and increasing, assisted
ventilation is continued and the neonate is observed. Failure of the heart rate
to rise above 80 beats/min is an indication for chest compres-sions.
Indications for endotracheal intubation include ineffective or prolonged mask
ventilation and the need to administer medications.
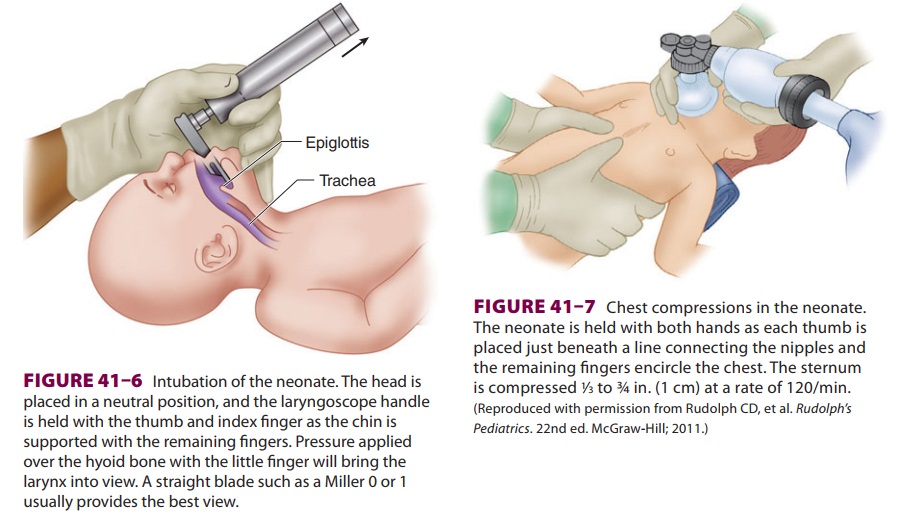
Intubation (Figure 41–6) is performed with a Miller 00, 0, or 1 laryngoscope blade, using a
2.5-, 3-, or 3.5-mm endotracheal tube (for neonates <1 kg, 1–2 kg, and >2 kg, respectively). Correct endotracheal
tube size is indicated by a small leak with 20 cm H2O pressure. Right endobronchial intubation
should be excluded by chest ausculta-tion. The correct depth of the
endotracheal tube (“tip to lip”) is usually 6 cm plus the weight in kilo-grams.
Oxygen saturation can usually be measured by a pulse oximeter probe applied to
the palm. Capnography is also very useful in confirming endotracheal
intubation. Transcutaneous oxygen sensors are useful for measuring tissue
oxygenation but require time for initial equilibration. Use of a laryngeal mask
airway (LMA#1) has been reported
in neonates weighing more than 2.5 kg and may be useful if endotracheal
intubation is difficult (eg, Pierre Robin syndrome).
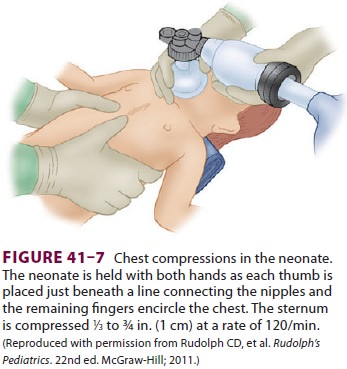
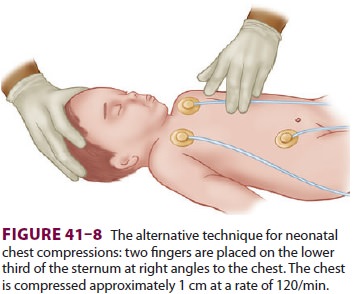
Guidelines for Chest Compressions
Indications for chest compressions are a heart rate that is less than 60
beats/min or 60–80 beats/min and not rising after 30 s of adequate ventilation
with 100% oxygen.
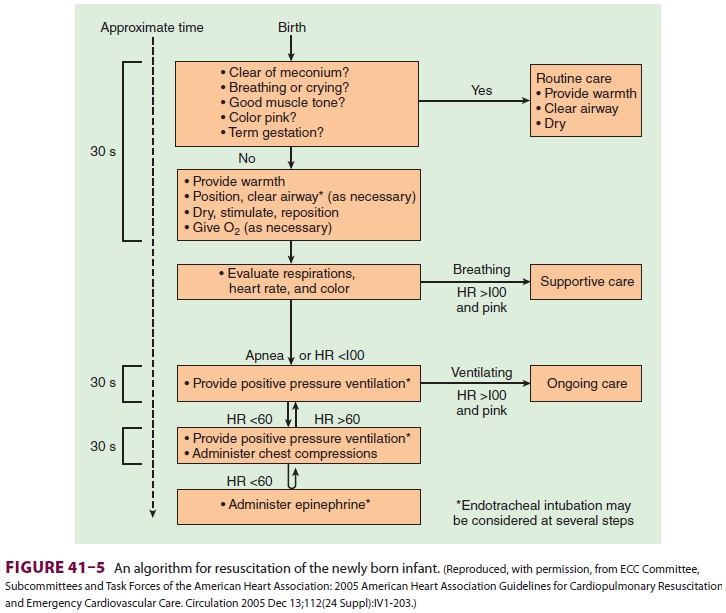
Cardiac compressions should be provided at a rate of 120/min. The two
thumb/encircling hands technique (Figure 41–7) is generally pre-ferred
because it appears to generate higher peak systolic and coronary perfusion
pressures. Alternatively, the two-finger technique can be used (Figure
41–8). The depth of compressions should be approximately one third of the
anterior–poste-rior diameter of the chest and enough to generate a palpable
pulse.
Compressions should be interposed with venti-lation in a 3:1 ratio, such
that 90 compressions and 30 ventilations are given per minute. The heart rate
should be checked periodically. Chest compressions should be stopped when the
spontaneous heart rate exceeds 80 beats/min.
Vascular Access
Cannulation of the umbilical vein with a 3.5F or 5F umbilical catheter
is easiest and the preferred tech-nique. The tip of the catheter should be just
below skin level and allow free backflow of blood; further advancement may
result in infusion of hypertonic solutions directly into the liver. A
peripheral vein or even the endotracheal tube can be used as an alter-nate
route for drug administration.
Cannulation of one of the two umbilical
arteries allows measurement of blood pressure and facilitates blood gas measurements
but may be more difficult. Specially designed umbilical artery catheters allow
continuous Pao2
or oxygen saturation monitoring as well as blood pressure. Care must be taken
not to introduce any air into either the artery or the vein.
Volume Resuscitation
Some neonates at term and nearly two thirds
of premature infants requiring resuscitation are hypo-volemic at birth.
Diagnosis is based on physical examination (low blood pressure and pallor) and
a poor response to resuscitation. Neonatal blood pres-sure generally correlates
with intravascular volume and should therefore routinely be measured. Normal
blood pressure depends on birth weight and varies from 50/25 mm Hg for neonates
weighing 1–2 kg to 70/40 mm Hg for those weighing over 3 kg. A low blood pressure
suggests hypovolemia. Volume expan-sion may be accomplished with 10 mL/kg of
lactated Ringer’s injection, normal saline, or type O-negative blood
cross-matched with maternal blood. Less common causes of hypotension include
hypocalce-mia, hypermagnesemia, and hypoglycemia.
Drug Therapy
A. Epinephrine
Epinephrine, 0.01–0.03 mg/kg (0.1–0.3 mL/kg
of a 1:10,000 solution), should be given for asystole or a spontaneous heart
rate of less than 60 beats/min in spite of adequate ventilation and chest compressions.
It may be repeated every 3–5 min. Epinephrine may be given in 1 mL of saline
via the endotracheal tube when venous access is not available.
B. Naloxone
Naloxone, 0.1 mg/kg intravenously or 0.2 mg/kg intramuscularly, is given
to reverse the respiratory depressant effect of opioids given to the mother in
the last 4 h of labor. Withdrawal symptoms may be precipitated in babies of
mothers who chronically consume prescribed or illicit opioids.
C. Other Drugs
Other drugs may be indicated only in specific settings. Sodium
bicarbonate (2 mEq/kg of a 0.5 mEq/mL 4.2% solution) should usually be given
only for a severe metabolic acidosis documented by blood gas measurements and
when ventilation is adequate. It may also be administered during prolonged resuscitation
(>5 min)—particularly if blood
gas measurements are not readily available. The infusion rate should not exceed
1 mEq/kg/min to avoid hypertonicity and intracranial hemorrhage. As noted
above, in order to prevent hypertonicity-induced hepatic injury, the umbilical
vein catheter tip should not be in the liver. Calcium gluconate mg/kg (CaCl 2, 30 mg/kg) should be given only to neonates with documented
hypocalcemia or those with suspected magnesium intoxication (from maternal
magnesium therapy); these neonates are usually hypotensive, hypotonic, and
appear vasodi-lated. Glucose (8 mg/kg/min of a 10% solution) is given only for
documented hypoglycemia because hyperglycemia worsens hypoxic neurological
defi-cits. Blood glucose should be measured because up to 10% of neonates may
have hypoglycemia (glucose <35 mg/dL), particularly those delivered by caesarean section. Dopamine
may be started at 5 mcg/kg/min to support arterial blood pressure. Lastly,
surfactant may be given through the endotracheal tube to pre-mature neonates
with respiratory distress syndrome.
Related Topics