Chapter: Clinical Anesthesiology: Anesthetic Management: Anesthesia for Patients with Respiratory Disease
Anesthesia : Asthma
ASTHMA
Preoperative Considerations
Asthma is a common disorder, affecting
5% to 7% of the population. Its primary characteristic is airway (bronchiolar)
inflammation and hyperreactivity in response to a variety of stimuli. Clinically,
asthma is manifested by episodic attacks of dyspnea, cough, and wheezing.
Airway obstruction, which is gener-ally reversible, is the result of bronchial
smooth mus-cle constriction, edema, and increased secretions.Classically, the
obstruction is precipitated by a vari-ety of airborne substances, including
pollens, animal dander, dusts, pollutants, and various chemicals. Some patients
also develop bronchospasm follow-ing ingestion of aspirin, nonsteroidal
antiinflam-matory agents, sulfites, or tartrazine and other dyes. Exercise,
emotional excitement, and viral infections also precipitate bronchospasm in
many patients. Asthma is classified as acute or chronic. Chronic asthma is
further classified as intermittent (mild) and mild, moderate, and severe persistent
disease.
The terms extrinsic (allergic) asthma
(attacks related to environmental exposures) and intrinsic (idiosyncratic)
asthma (attacks usually occurring without provocation) were used in the past,
but these classifications were imperfect; many patients show features of both
forms. Moreover, overlap with chronic bronchitis is common.
A. Pathophysiology
The
pathophysiology of asthma involves the local release of various chemical
mediators in the airway, and, possibly, overactivity of the parasympathetic
nervous system. Inhaled substances can initiate bronchospasm through both
specific and nonspecific immune mechanisms by degranulating bronchial mast
cells. In classic allergic asthma, antigen binding to immunoglobulin E (IgE) on
the surface of mast cells causes degranulation. Bronchoconstriction is the
result of the subsequent release of histamine; bra-dykinin; leukotrienes C, D,
and E; platelet-activating factor; prostaglandins (PG) PGE2, PGF2α,
and PGD2; and neutrophil and eosinophil chemotactic factors. The
parasympathetic nervous system plays a major role in maintaining normal
bronchial tone; a normal diurnal variation in tone is recognized in most
individuals, with peak airway resistance occur-ring early in the morning (at
about 6:00 am). Vagal afferents in the bronchi are sensitive to histamine and
multiple noxious stimuli, including cold air, inhaled irritants, and
instrumentation (eg, tra-cheal intubation). Reflex vagal activation resultsin
bronchoconstriction, which is mediated by an increase in intracellular cyclic
guanosine mono-phosphate (cGMP). During an asthma attack, bronchoconstriction,
mucosal edema, and secretions increase resistance to gas flow at all levels of
the lower airways. As an attack resolves, airway resistance normalizes first in
the larger airways (main-stem, lobar, segmental, and subsegmental bronchi), and
then in more peripheral airways. Consequently, expiratory flow rates are
initially decreased throughout an entire forced exhalation, but during
resolution of the attack, the expiratory flow rate is reduced only at low lung
volumes. TLC, residual volume (RV), and FRC are all increased. In acutely ill
patients, RV and FRC are often increased by more than 400% and 100%,
respectively. Prolonged or severe attacks markedly increase the work of
breathing and can fatigue respiratory· muscles·. The number of alveolar units
with low (V/Q) ratios increases, resulting in hypoxemia. Tachypnea is likely
due to stimulation of bronchial receptors and typically produces hypocapnia. A
normal or high Paco2 indicates that the patient can no longer
maintain the work ofbreathing and is often a sign of impending respira-tory
failure. A pulsus paradoxus and electrocardio-graphic signs of right
ventricular strain (ST-segment changes, right axis deviation, and right bundle
branch block) are also indicative of severe airway obstruction.
B. Treatment
Drugs
used to treat asthma include β-adrenergic
agonists, methylxanthines, glucocorticoids, anti-cholinergics, leukotriene
blockers, and mast cell-stabilizing agents; with the exception of the last,
these drugs may be used for either acute or chronic treatment of asthma.
Although devoid of any bron-chodilating properties, cromolyn sodium and
nedo-cromil are effective in preventing bronchospasm by blocking the
degranulation of mast cells.Sympathomimetic agents ( Table24–3)
are the most commonly used for acute exacerbations. They produce bronchodilation
via β2-agonist
activ-ity. Activation of β2-adrenergic
receptors on bron-chiolar smooth muscle stimulates the activity of adenylate
cyclase, which results in the formation of intracellular cyclic adenosine
monophosphate (cAMP). These agents are usually administered via a metered-dose
inhaler or by aerosol. Use of more selective β2-agonists,
such as terbutaline or alb-uterol, may decrease the incidence of undesirable β1
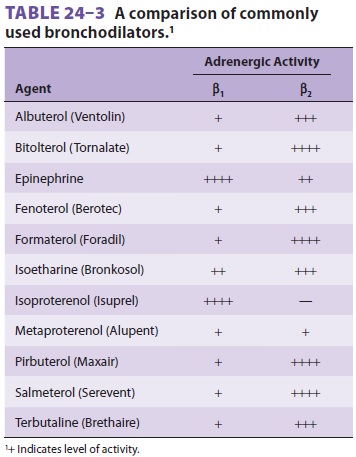
cardiac
effects, but are often not particularly selec-tive in high doses.
Traditionally,
methylxanthines are thought to produce bronchodilation by inhibiting
phosphodi-esterase, the enzyme responsible for the breakdown of cAMP. Their
pulmonary effects seem much more complex and include catecholamine release,
block-ade of histamine release, and diaphragmatic stimu-lation. Oral
long-acting theophylline preparations are used for patients with nocturnal
symptoms. Unfortunately, theophylline has a narrow therapeu-tic range;
therapeutic blood levels are considered to be 10–20 mcg/mL. Lower levels,
however, may be effective. Aminophylline is the only available intra-venous
theophylline preparation.
Glucocorticoids
are used for both acute treat-ment and maintenance therapy of patients with
asthma because of their antiinflammatory and membrane-stabilizing effects.
Beclomethasone, tri-amcinolone, fluticasone, and budesonide are syn-thetic
steroids commonly used in metered-dose inhalers for maintenance therapy.
Although they are associated with a low incidence of undesirable systemic eff
ects, their use does not necessarily prevent adrenal suppression. Intravenous
hydrocortisone or methylprednisolone is used acutely for severe attacks,
followed by tapering doses of oral prednisone. Glucocorticoids usually require
several hours to become eff ective.
Anticholinergic
agents produce bronchodilation through their antimuscarinic action and may
block refl ex bronchoconstriction. Ipratropium, a congener of atropine that can
be given by a metereddose inhaler or aerosol, is a moderately eff ective
bronchodilator without appreciable systemic anticholinergic effects.
Anesthetic Considerations
A. Preoperative Management
The
emphasis in evaluating patients with asthma should be on determining the recent
course of the disease and whether the patient has ever been hospi-talized for
an acute asthma attack, as well as on ascertaining that the patient is in
optimal condition. Patients with poorly controlled asthma or wheezing at the
time of anesthesia induction have a higher risk of perioperative complications.
Conversely, well-controlled asthma has not been shown to be a risk factor for
intraoperative or postoperative complica-tions. A thorough history and physical
examination are of critical importance. The patient should have no or minimal
dyspnea, wheezing, or cough. Com-plete resolution of recent exacerbations
should be confirmed by chest auscultation. Patients with fre-quent or chronic
bronchospasm should be placed on an optimal bronchodilating regimen. A chest
radio-graph identifies air trapping; hyperinflation results in a flattened
diaphragm, a small-appearing heart, and hyperlucent lung fields.
PFTs—particularly expiratory airflow measurements such as FEV1,
FEV1/FVC,
FEF25-75%, and peak expiratory flow rate—help in assessing the
severity of airwayobstruction and reversibility after bronchodilator treatment.
Comparisons with previous measure-ments are invaluable.Asthmatic patients with
active bronchospasm presenting for emergency surgery should betreated
aggressively. Supplemental oxygen, aerosol-ized β2-agonists,
and intravenous glucocorticoids can dramatically improve lung function in a few
hours. Arterial blood gases may be useful in man-aging severe cases. Hypoxemia
and hypercapnia are typical of moderate and severe disease; even slight
hypercapnia is indicative of severe air trapping and may be a sign of impending
respiratory failure.Some degree of preoperative sedation may be desirable in
asthmatic patients presenting for elec-tive surgery—particularly in patients
whose disease has an emotional component. In general, benzodiaz-epines are the
most satisfactory agents for premedi-cation. Anticholinergic agents are not
customarily given unless very copious secretions are present or if ketamine is
to be used for induction of anesthesia. In typical intramuscular doses,
anticholinergics are not effective in preventing reflex bronchospasm fol-lowing
intubation. The use of an H2-blocking agent (such as cimetidine,
ranitidine, or famotidine) is the-oretically detrimental, since H2-receptor
activation normally produces bronchodilation; in the event of histamine
release, unopposed H1 activation with H2 blockade may
accentuate bronchoconstriction.Bronchodilators should be continued up to the
time of surgery; in order of effectiveness, they are β-agonists,
inhaled glucocorticoids, leukotri-ene blockers, mast-cell stabilizers,
theophyllines, and anticholinergics. Patients who receive chronic
glucocorticoid therapy with more than 5 mg/day of prednisone (or its
equivalent) should receive a graduated supplementation schedule based on the
severity of the illness and complexity of the surgical procedure. Supplemental
doses should be tapered to baseline within 1–2 days.
B. Intraoperative Management
The most critical time for asthmatic
patients under-going anesthesia is during instrumentation of the airway.
General anesthesia by mask or regional anesthesia will circumvent this problem,
but nei-ther eliminates the possibility of bronchospasm. In fact, some
clinicians believe that high spinal or epi-dural anesthesia may aggravate
bronchoconstriction by blocking sympathetic tone to the lower airways (T1–T4)
and allowing unopposed parasympathetic activity. Pain, emotional stress, or
stimulation dur-ing light general anesthesia can precipitate bron-chospasm.
Drugs often associated with histamine release (eg, atracurium, morphine, and
meperidine) should be avoided or given very slowly when used. The goal of any
general anesthetic is a smooth induc-tion and emergence, with anesthetic depth
adjusted to stimulation.
The choice of induction agent is less
important, if adequate depth of anesthesia is achieved before intubation or
surgical stimulation. Thiopental may occasionally induce bronchospasm as a
result of exaggerated histamine release. Propofol and etomi-date are suitable
induction agents; propofol may also produce bronchodilation. Ketamine has
bron-chodilating properties and is a good choice for patients with asthma who
are also hemodynamically unstable. Ketamine should probably not be used in
patients with high theophylline levels, as the com-bined actions of the two
drugs can precipitate seizure activity. Halothane and sevoflurane usually
provide the smoothest inhalation induction with bronchodi-lation in asthmatic
children. Isoflurane and desflu-rane can provide equal bronchodilation, but are
not normally used for inhalation induction. Desflurane is the most pungent of
the volatile agents and may result in cough, laryngospasm, and bronchospasm.
Reflex bronchospasm can be blunted
before intubation by an additional dose of the induction agent, ventilating the
patient with a 2–3 minimum alveolar concentration (MAC) of a volatile agent for
5 min, or administering intravenous or intra-tracheal lidocaine (1–2 mg/kg).
Note that intra-tracheal lidocaine itself can initiate bronchospasm if an
inadequate dose of induction agent has been used. Administration of an
anticholinergic agent may block reflex bronchospasm, but causes exces-sive
tachycardia. Although succinylcholine may on occasion induce marked histamine release,
it can generally be safely used in most asthmatic patients. In the absence of
capnography, confirmation of cor-rect tracheal placement by chest auscultation
can be difficult in the presence of marked bronchospasm.
Volatile anesthetics are most often used
for maintenance of anesthesia to take advantage of their potent bronchodilating
properties. Ventilation should incorporate warmed humidified gases whenever
possible. Airflow obstruction during expiration is apparent on capnography as a
delayed rise of the end-tidal CO 2 value ( Figure24–2); the
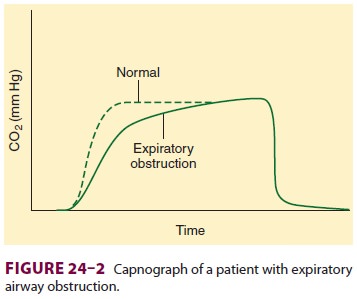
severity of obstruction is generally
inversely related to the rate of rise in end-tidal CO 2. Severe broncho-spasm is manifested by rising peak
inspiratory pres-sures and incomplete exhalation. Tidal volumes of 6–8 mL/kg,
with prolongation of the expiratory time, may allow more uniform distribution
of gas flow to both lungs and may help avoid air trapping. The Paco2 may increase, which is acceptable if there is no
contraindication from a cardiovascular or neurologic perspective.Intraoperative
bronchospasm is usually mani-fested as wheezing, increasing peak
airwaypressures (plateau pressure may remain unchanged), decreasing exhaled
tidal volumes, or a slowly risingwaveform on the capnograph. Other causes can
simulate bronchospasm: obstruction of
the tracheal tube from kinking,
secretions, or an overinflated balloon; bronchial intubation; active expiratory
efforts (straining); pulmonary edema or embolism; and pneumothorax. Bronchospasm
should be treated by increasing the concentration of the volatile agent and
administering an aerosolized bronchodilator. Infusion of low dose epinephrine
may be needed if bronchospasm is refractory to other interventions.Intravenous
hydrocortisone can be given, par-ticularly in patients with a history of
glucocorticoid therapy.
At the completion of surgery, the
patient should ideally be free of wheezing. Reversal of nondepolarizing
neuromuscular blocking agents with anticholinesterase agents does not
precipitate bronchoconstriction, if preceded by the appropri-ate dose of an
anticholinergic agent. Deep extu-bation (before airway reflexes return) reduces
bronchospasm on emergence. Lidocaine as a bolus (1.5–2 mg/kg) may help obtund
airway reflexes dur-ing emergence.
Related Topics