Examples, Preparation, Properties, Uses - Alums | 12th Chemistry : UNIT 2 : p-Block Elements-I
Chapter: 12th Chemistry : UNIT 2 : p-Block Elements-I
Alums
Alums:
The name alum is given
to the double salt of potassium aluminium sulphate [K2SO4.
Al2(SO4)3.24.H2O]. Now a days it is
used for all the double salts with M'2SO4.M"2(SO4)3.24H2O,
where M' is univalent metal ion or [NH4]+ and M" is
trivalent metal ion.
Examples:
Potash alum [K2SO4.Al2(SO4)3.24.H2O];
Sodium alum [Na2SO4.Al2(SO4)3.24.H2O]
, Ammonium alum [(NH4)2SO4.Al2(SO4)3.24.H2O],
Chrome alum [K2SO4.Cr2(SO4)3.24.H2O].
Alums in general are
more soluble in hot water than in cold water and in solutions they exhibit the
properties of constituent ions.
Preparation:
The alunite the alum
stone is the naturally occurring form and it is K2SO4. Al2(SO4)3.4Al(OH)3.
When alum stone is treated with excess of sulphuric acid, the aluminium
hydroxide is converted to aluminium sulphate. A calculated quality of potassium
sulphate is added and the solution is crystallised to generate potash alum. It
is purified by recrystallisation.
K2SO4.Al2(SO4)3
.4Al(OH)3 + 6H2SO4 → K2SO4 + 3Al2(SO4)3
+ 12 H2O
K2SO4
+ Al2(SO4)3 + 24 H2O →
K2SO4.Al2(SO4)3.24 H2O
Properties
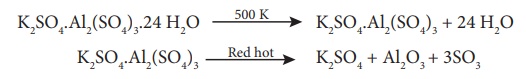
Potash alum is a white
crystalline solid it is soluble in water and insoluble in alcohol. The aqueous
solution is acidic due to the hydrolysis of aluminium sulphate it melts at 365
K on heating. At 475 K loses water of hydration and swells up. The swollen mass
is known as burnt alum. Heating to red hot it decomposes into potassium
sulphate, alumina and sulphur-trioxide.
Potash alum forms
aluminium hydroxide when treated with ammonium hydroxide.
K2SO4.Al2(SO4)3.24
H2O +6NH4OH → K2SO4
+ 3(NH4)2SO4 + 24 H2O + 3Al(OH)3
Uses of Alum:
·
It is used for purification of water
·
It is also used for water proofing and textiles
·
It is used in dyeing, paper and leather tanning industries
·
It is employed as a styptic agent to arrest bleeding.
Related Topics