Chapter: Organic Chemistry: Alkanes and cycloalkanes
Alkanes and cycloalkanes: Nomenclature
NOMENCLATURE
Key Notes
Simple alkanes
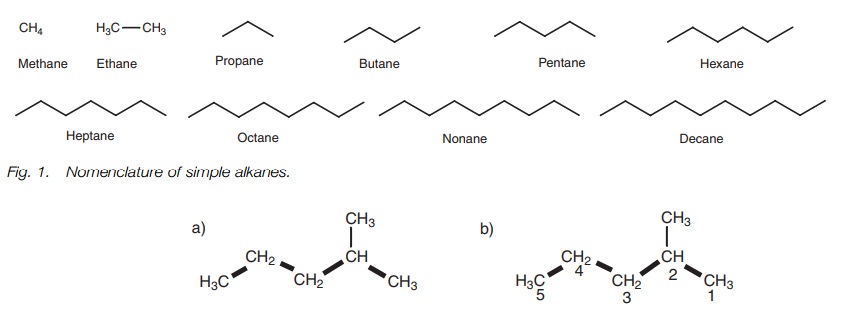
The
names of the first 10 simple alkanes are methane, ethane, propane, butane,
pentane, hexane, heptane, octane, nonane, and decane.
Branched alkanes
Branched
alkanes have alkyl substituents branching off from the main chain. When naming
a branched alkane, identify the longest chain and number it from the end
nearest the branch point. Identify the substituent and its position on the
longest chain. The name is n-alkylalkane
where n is the position of the
substituent, alkyl is the substituent and alkane is the longest chain.
Multi-branched alkanes
If there
is more than one substituent present, the substituents are named in
alphabetical order. Identical substituents are identified by prefixing them
with di-, tri-, tetra-, etc., but the order of naming still depends on the
alpha-betical order of the substituents themselves. If there are two different
sub-stituents at equal distances from either end of the chain, the substituent
with alphabetical priority has the lowest numbering. This rule may be
sup-planted if there are several substituents so placed.
Cycloalkanes
Cycloalkanes
are named according to the number of carbon atoms making up the ring, that is,
cyclopropane (C3H6), cyclobutane (C4H8),
cyclopentane (C5H10), cyclohexane (C6H12),
etc.
Branched cycloalkanes
Cycloalkanes
linked to an alkane are usually named such that the cycloalkane is considered
the parent system and the alkane group is an alkyl substituent (i.e.
alkylcycloalkane). However, the opposite holds true if the alkane portion has
more carbon atoms than the cycloalkane in which case the cycloalkane is
considered a substituent of the alkane portion (i.e. n-cycloalkylalkane).
Multi-branched cycloalkanes
Cycloalkanes
having several substituents are numbered such that the sub-stituent with
alphabetical priority is at position 1. Numbering is then car-ried out such
that the total obtained from the substituent positions is a minimum.
Simple alkanes
The names of the simplest straight chain
alkanes are shown in Fig. 1.
Branched alkanes
Branched alkanes are alkanes with alkyl
substituents branching off from the main chain. They are named by the following
procedure:
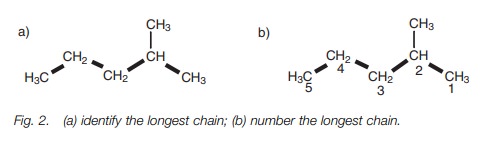
·
identify the longest chain of carbon atoms. In the example shown (Fig. 2a), the longest chain consists of five
carbon atoms and a pentane chain;
·
number the longest chain of carbons, starting from the end nearest
the branch point (Fig. 2b);
·
identify the carbon with the branching group (number 2 in Fig. 2b);
·
identify and name the branching group. (In this example it is CH3.
Branching groups (or substituents)
are referred to as alkyl groups (CnH2n1) rather than alkanes (CnH2n2). Therefore, CH3 is
called methyl and not methane.)
·
name the structure by first identifying the substituent and its
position in the chain, then naming the longest chain. The structure in Fig. 1 is called 2-methylpentane. Notice
that the substituent and the main chain is one complete word, that is,
2-methylpentane rather than 2-methyl pentane.
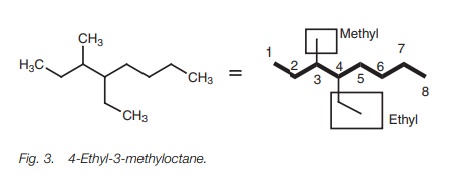
Multi-branched alkanes
If
there is more
than one alkyl
substituent present in the structure
then the substituents are named
in alphabetical order, numbering again from the end of the chain nearest the
substituents. The structure in Fig. 3
is 4-ethyl-3-methyloctane and not 3-methyl-4-ethyloctane.
If a structure has identical substituents, then
the prefixes di-, tri-, tetra-, et ceteraare
used to represent the number of substituents. For example, the structure in Fig. 4 is called 2,2-dimethylpentane and
not 2-methyl-2-methylpentane.

The prefixes di-, tri-, tetra- etc. are used
for identical substituents, but the order in which they are written is still
dependent on the alphabetical order of the substituents themselves (i.e. ignore
the di-, tri-, tetra-, et cetera).
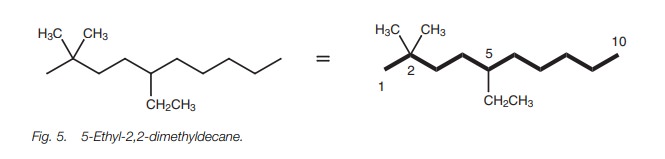
For example, the structure in Fig. 5 is
called 5-ethyl-2,2-dimethyldecane and not 2,2-dimethyl-5-ethyldecane.
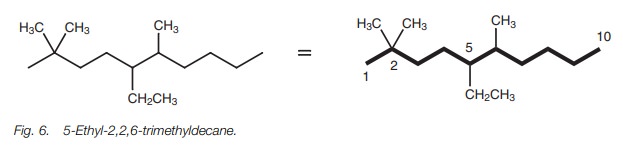
Identical substituents can be in different
positions on the chain, but the same rules apply. For example, the structure in
Fig. 6 is called
5-ethyl-2,2,6-trimethyldecane.
In some structures, it is difficult to decide
which end of the chain to number from. For example, two different substituents
might be placed at equal distances from either end of the chain. If that is the
case, the group with alphabetical prior-ity should be given the lowest
numbering. For example, the structure in Fig.
7a is 3-ethyl-5-methylheptane and not 5-ethyl-3-methylheptane.
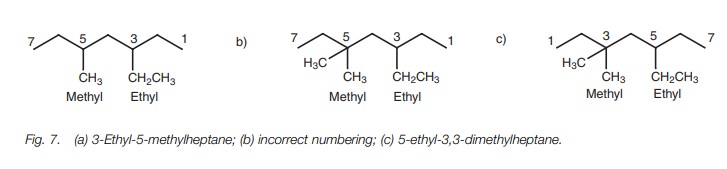
However, there is another rule which might take
precedence over the above rule. The structure (Fig. 7c) has ethyl and methyl groups equally placed from each end
of the chain, but there are two methyl groups to one ethyl group. Num-bering
should be chosen such that the smallest total is obtained. In this example, the
structure is called 5-ethyl-3,3-dimethylheptane (Fig. 7c) rather than 3-ethyl-5,5-dimethylheptane (Fig. 7b) since 5 3 3 = 11 is less than 3
5 5 = 13.
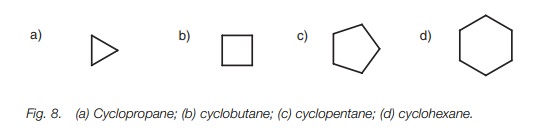
Cycloalkanes
Cycloalkanes are simply named by identifying
the number of carbons in the ring and prefixing the alkane name with cyclo (Fig. 8).
Branched cyclohexanes
Cycloalkanes consisting of a cycloalkane moiety
linked to an alkane moiety are usually named such that the cycloalkane is the
parent system and the alkane moiety is considered to be an alkyl substituent.
Therefore, the structure in Fig. 9a
is methylcyclohexane and not cyclohexylmethane. Note that there is no need to
number the cycloalkane ring when only one substituent is present.
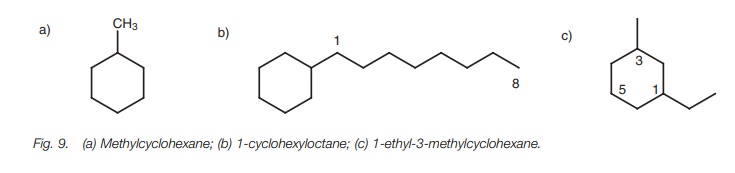
If the alkane moiety contains more carbon atoms
than the ring, the alkane moiety becomes
the
parent system and
the cycloalkane group
becomes the substituent. For
example, the structure in Fig. 9b is
called 1-cyclohexyloctane and not octylcyclohexane. In this case, numbering is
necessary to identify the position of the cycloalkane on the alkane chain.
Multi-branched cycloalkanes
Branched cycloalkanes having different
substituents are numbered such that the alkyl substituent having alphabetical
priority is at position 1. The numbering of the rest of the ring is then
carried out such that the substituent positions add up to a minimum. For
example, the structure in Fig. 9c is
called 1-ethyl-3-methyl-cyclohexane rather than 1-methyl-3-ethylcyclohexane or
1-ethyl-5-methylcyclo-hexane. The last name is incorrect since the total
obtained by adding the substituent positions together is 5 1 6 which is higher
than the total obtained from the correct name (i.e. 1 3 4)
Related Topics