Chapter: Engineering Chemistry: Surface Chemistry and Catalysis
Adsorption and Absorption
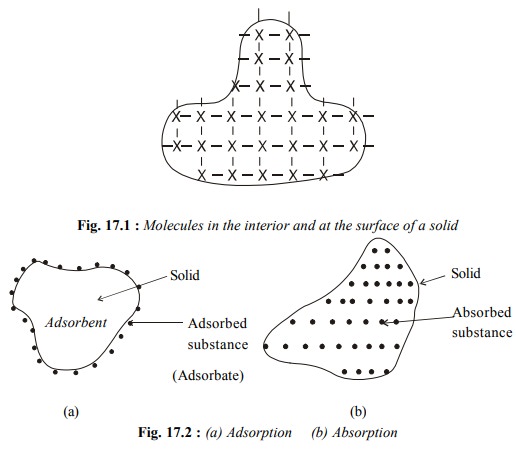
The phenomenon of adsorption is different from that of absorption.
Adsorption
and Absorption
The phenomenon of adsorption is different from that of absorption. The latter term implies that a substance is uniformly
distributed throughout the body of a solid, Fig. 17.2(b). If we leave a small
lump of calcium chloride in open, it absorbs water vapour (moisture) from air
and after some time even starts dissolving in it. On the other hand if we keep
a sample of silica gel in open, it adsorbs water vapour on its surface as shown
in Fig. 17.2 (a).
Study Material, Lecturing Notes, Assignment, Reference, Wiki description explanation, brief detail
Engineering Chemistry: Surface Chemistry and Catalysis : Adsorption and Absorption |
Related Topics
Engineering Chemistry: Surface Chemistry and Catalysis