Chapter: Medical Physiology: Regulation of Acid-Base Balance
Acids and Bases-Their Definitions and Meanings
Acids and Bases—Their Definitions and Meanings
A hydrogen ion is a single free proton released from a hydrogen atom. Molecules containing hydrogen atoms that can release hydrogen ions in solutions are referred to as acids. An example is hydrochloric acid (HCl), which ionizes in water to form hydrogen ions (H+) and chloride ions (Cl–). Likewise, carbonic acid (H2CO3) ionizes in water to form H+ and bicarbonate ions (HCO3–).
A base is an ion or a molecule that can accept an H+. For example, HCO3– is a base because it can combine with H+ to form H2CO3. Likewise, HPO4= is a base because it can accept an H+ to form H2PO4–. The proteins in the body also function as bases, because some of the amino acids that make up proteins have net negative charges that readily accept H+. The protein hemoglobin in the red blood cells and proteins in the other cells of the body are among the most important of the body’s bases.
The terms base and alkali are often used synonymously. An alkali is a molecule formed by the combination of one or more of the alkaline metals—sodium, potas-sium, lithium, and so forth—with a highly basic ion such as a hydroxyl ion (OH–).
The base portion of these molecules reacts quickly with H+ to remove it from solution; they are, therefore, typical bases. For similar reasons, the term alkalosis refers to excess removal of H+ from the body fluids, in contrast to the excess addition of H+, which is referred to as acidosis.
Strong and Weak Acids and Bases. A strong acid is one thatrapidly dissociates and releases especially large amounts of H+ in solution. An example is HCl. Weak acids have less tendency to dissociate their ions and, therefore, release H+ with less vigor. An example is H2CO3. A strong base is one that reacts rapidly and strongly with H+ and, therefore, quickly removes these from a solution. A typical example is OH–, which reacts with H+ to form water (H2O). A typical weak base is HCO3– because it binds with H+ much more weakly than does OH–. Most of the acids and bases in the extracel-lular fluid that are involved in normal acid-base regula-tion are weak acids and bases. The most important ones that we discuss in detail are H2CO3 and bicarbonate base.
Normal Hydrogen Ion Concentration and pH of Body Fluids and Changes That Occur in Acidosis and Alkalosis. As dis-cussed earlier, the blood H+ concentration is normally maintained within tight limits around a normal value of about 0.00004 mEq/L (40 nEq/L). Normal variations are only about 3 to 5 nEq/L, but under extreme conditions, the H+ concentration can vary from as low as 10 nEq/L to as high as 160 nEq/L without causing death.
Because H+ concentration normally is low, and because these small numbers are cumbersome, it is cus-tomary to express H+concentration on a logarithm scale, using pH units. pH is related to the actual H+ con-centration by the following formula (H+concentration [H+] is expressed in equivalents per liter):
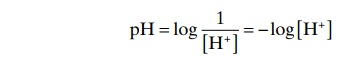
For example, the normal [H+] is 40 nEq/L (0.00000004 Eq/L). Therefore, the normal pH is
pH = –log [0.00000004]
pH = 7.4
From this formula, one can see that pH is inversely related to the H+ concentration; therefore, a low pH corresponds to a high H+ concentration, and a high pH corresponds to a low H+ concentration.
The normal pH of arterial blood is 7.4, whereas the pH of venous blood and interstitial fluids is about 7.35 because of the extra amounts of carbon dioxide (CO2) released from the tissues to form H2CO3 in these fluids (Table 30–1). Because the normal pH of arterial blood is 7.4, a person is considered to have acidosis when the pH falls below this value and to have alkalosis when the pH rises above 7.4. The lower limit of pH at which a person can live more than a few hours is about 6.8, and the upper limit is about 8.0.
Intracellular pH usually is slightly lower than plasma pH because the metabolism of the cells produces acid, especially H2CO3. Depending on the type of cells, the pH of intracellular fluid has been estimated to range between 6.0 and 7.4. Hypoxia of the tissues and poor blood flow to the tissues can cause acid accumulation and decreased intracellular pH.
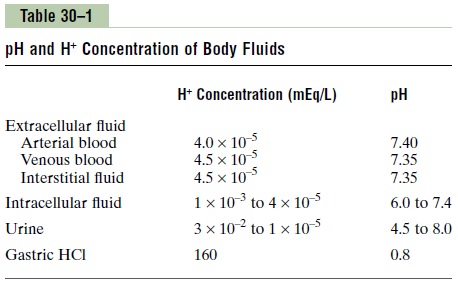
The pH of urine can range from 4.5 to 8.0, depending on the acid-base status of the extracellular fluid. As dis-cussed later, the kidneys play a major role in correcting abnormalities of extracellular fluid H+ concentration by excreting acids or bases at variable rates.
An extreme example of an acidic body fluid is the HCl secreted into the stomach by the oxyntic (parietal) cells of the stomach mucosa. The H+ concentration in these cells is about 4 million times greater than the hydrogen concentration in blood, with a pH of 0.8.
Related Topics