Chapter: Organic Chemistry: Acid–base reactions
Acid strength
ACID STRENGTH
Key Notes
Electronegativity
The
acidity of protons depends on the electronegativity of the atoms to which they
are attached. The more electronegative the atom, the more acidic the proton
will be. Therefore, a hydrogen atom attached to a halogen atom will be more
acidic than a hydrogen atom attached to oxygen. A hydrogen atom attached to
oxygen will be more acidic than a hydrogen atom attached to nitrogen. Hydrogen
atoms attached to carbon are not usually acidic at all.
pKa
pKa is a measure of the
strength of an acid. The lower the value of pKa the stronger the acid.pKa is the negative logarithm of Ka which is a measure of the dissociation or ionization
of the acid. The larger the value of Ka,
the stronger the acid.
Inductive effects
Inductive
effects can affect the stability of the conjugate base by stabilizing or
destabilizing the negative charge. Electron-withdrawing groups such as halogens
diminish the charge and stabilize the conjugate base, resulting in a stronger
acid. Electron-donating groups (e.g. alkyl groups) will increase the charge and
destabilize the conjugate base, resulting in a weaker acid.
Resonance
A
negative charge can be stabilized by resonance, resulting in delocalization of
the charge over two or more atoms. Carboxylic acids are acidic because the
resulting carboxylate ion can be stabilized by delocalization of the charge
between two oxygen atoms. Phenols are acidic because the resulting phenolate
ion can be stabilized by delocalization of the charge between the oxygen and
three carbon atoms. Alcohols are only weakly acidic because the charge on the
resulting alkoxide ion is localized on the oxygen and destabilized by the
inductive effect of the alkyl group.
Amines and amides
Amines
and amides are very weak acids. However, amides are more acidic than amines due
to resonance and inductive effects.
Electronegativity
The acidic protons of various molecules are not
equally acidic and their relative acidity depends on a number of factors, one
of which is the electronegativity of the atom to which they are attached. For
example, consider hydrofluoric acid, ethanoic acid, and methylamine (Fig. 1). Hydrofluoric acid has the most
acidic proton since the hydrogen is attached to a stronglyelectronegative
fluorine. The fluorine strongly polarizes the H–F bond such that the hydrogen
becomes highly electron deficient and is easily lost. Once the proton is lost,
the fluoride ion can stabilize the resulting negative charge.
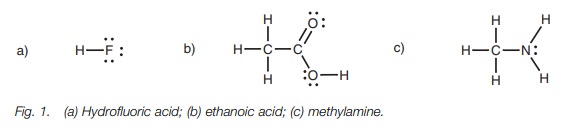
The acidic protons on methylamine are attached
to nitrogen which is less elec-tronegative than fluorine. Therefore, the N–H
bonds are less polarized, and the protons are less electron deficient. If one
of the protons is lost, the nitrogen is left with a negative charge which it
cannot stabilize as efficiently as a halide ion. All of this means that
methylamine is a much weaker acid than hydrogen fluoride.
Ethanoic acid is more acidic than methylamine
but less acidic than hydrofluoric acid. This is because the electronegativity
of oxygen lies between that of a halogen and that of a nitrogen atom.
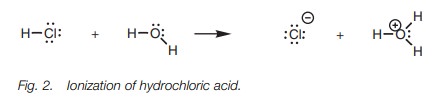
These differences in acid strength can be demonstrated
if the three molecules above are placed in water. Mineral acids such as HF,
HCl, HBr, and HI are strong acids and dissociate
or ionize completely (Fig. 2).
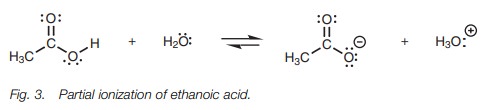
Ethanoic acid (acetic acid) partially
dissociates in water and an equilibrium is set up between the carboxylic acid
(termed the free acid) and the
carboxylate ion (Fig. 3). An acid
which only partially ionizes in this manner is termed a weak acid.
If methylamine is dissolved in water, none of
the acidic protons are lost at all and the amine behaves as a weak base instead
of an acid, and is in equilibrium with its protonated form (Fig. 4).
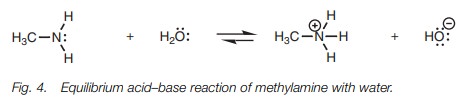
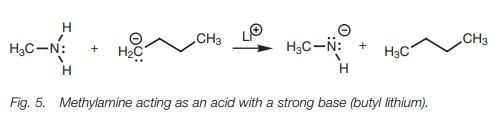
Methylamine can act as an acid but it has to be
treated with a strong base such as butyl lithium (Fig. 5).
Lastly, hydrogen atoms attached to carbon are
not usually acidic since carbon atoms are not electronegative. There are
exceptions to this rule.
pKa
Acids can be described as being weak or strong
and the pKa is a measure of this.
Dissolving acetic acid in water, results in an equilibrium between the
carboxylic acid and the carboxylate ion (Fig. 6).
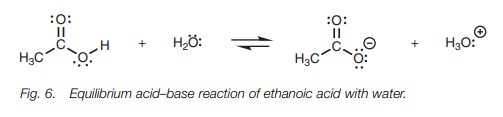
Ethanoic acid on the left hand of the equation
is termed the free acid, while the carboxylate ion formed on the right hand
side is termed its conjugate base. The extent of ionization or dissociation is
defined by the equilibrium constant (Keq
);
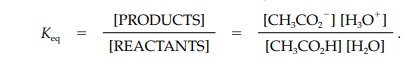
Keq is
normally measured in a dilute aqueous solution of the acid and so the
concentration of water is high and assumed to be constant. Therefore, we can
rewrite the equilibrium equation in a simpler form where Ka is the acidity con- stant and includes the
concentration of pure water (55.5 M).
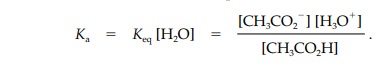
The acidity constant is also a measure of
dissociation and of how acidic a particu-lar acid is. The stronger the acid,
the more it is ionized and the greater the con-centration of products in the
above equation. This means that a strong acid has a high Ka value. The Ka
values for the following ethanoic acids are in brackets and demonstrate that
the strongest acid in the series is trichloroacetic acid.
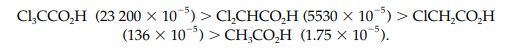
Kavalues
are awkward to work with and so it is more usual to measure theacidic strength
as a pKa value rather than
Ka. The pKa is the negative logarithm
of Ka (pKa = - log10Ka) and results in more
manageable numbers. The pKavalues
for each of the above ethanoic acids is shown in brackets below. The strongest
acid (trichloroacetic acid) has the lowest pKa
value.
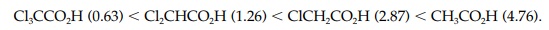
Therefore the stronger the acid, the higher the value of Ka, and the lower the value of pKa. An amine such as
ethylamine (CH3CH2NH2) is an extremely weak
acid (pKa = 40) compared
to ethanol (pKa = 16).
This is due to the relative electronega-tivities of oxygen and nitrogen as
described above. However, the electronegativ-ity of neighboring atoms is not
the only influence on acidic strength. For example, the pKa values of ethanoic acid (4.76), ethanol (16), and
phenol (10) show that ethanoic acid is more acidic than phenol, and that phenol
is more acidic than ethanol. The difference in acidity is quite marked, yet
hydrogen is attached to oxygen in all three structures.
Similarly, the ethanoic acids Cl3CCO2H
(0.63), Cl2CHCO2H (1.26), ClCH2CO2H
(2.87), and CH3CO2H (4.76) have significantly different pKa values and yet the acidic
hydrogen is attached to an oxygen in each of these structures. Therefore,
factors other than electronegativity have a role to play in determining acidic
strength.
Inductive effects
Stabilizing the negative charge of the
conjugate base is important in determining the strength of the acid and so any
effect which stabilizes the charge will result in a stronger acid. Substituents
can help to stabilize a negative charge and do so by an inductive effect. This is illustrated by comparing the pKa values of the alcohols CF3CH2OH
and CH3CH2OH (12.4 and 16, respectively) where CF3CH2OH
is more acidic than CH3CH2OH. This implies that the anion
CF3CH2O is more stable than CH3CH2O
(Fig. 7).
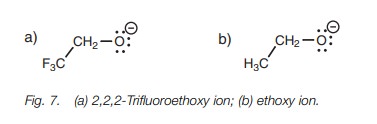
Fluorine atoms are strongly electronegative and
this means that each C–F bond is strongly polarized such that the carbon
bearing the fluorine atoms becomes strongly electropositive. Since this carbon
atom is now electron deficient, it will ‘demand’ a greater share of the
electrons in the neighboring C–C bond. This results in electrons being
withdrawn from the neighboring carbon, making it elec-tron deficient too. This
inductive effect will continue to be felt through the various bonds of the
structure. It will decrease through the bonds but it is still significant
enough to be felt at the negatively charged oxygen. Since the inductive effect
is electron withdrawing it will decrease the negative charge on the oxygen and
help to stabilize it. This means that the original fluorinated alcohol will
lose its proton more readily and will be a stronger acid.
This inductive effect explains the relative
acidities of the chlorinated ethanoic acids Cl3CCO2H
(0.63), Cl2CHCO2H (1.26), ClCH2CO2H
(2.87), and CH3CO2H (4.76). Trichloroethanoic acid is the
strongest acid since its conjugate base (the carboxylate ion) is stabilized by
the inductive effect created by three electronegative chlorine atoms. As the
number of chlorine atoms decrease, so does the inductive effect .
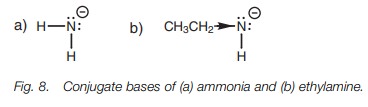
Inductive effects also explain the difference
between the acid strengths of ethylamine (pKa
~ 40) and ammonia (pKa ~
33). The pKa values
demonstrate that ammonia is a stronger acid than ethylamine. In this case, the
inductive effect is electron donating. The alkyl group of ethylamine enhances
the negative charge of the conjugate base and so destabilizes it, making
ethylamine a weaker acid than ammonia (Fig.
8).
Resonance
The negative charge on some conjugate bases can
be stabilized by resonance.
Resonance involves the movement of valence electrons around a structure, resulting
in the sharing of charge between different atoms – a process called delocalization. The effects of
resonance can be illustrated by comparing theacidities of ethanoic acid (pKa 4.76), phenol (pKa 10.0) and ethanol (pKa 12.4). The pKa values illustrate that
ethanoic acid is a stronger acid than phenol, and that phenol is a stronger
acid than ethanol.
The differing acidic strengths of ethanoic
acid, phenol and ethanol can be explained by considering the relative
stabilities of their conjugate bases (Fig.
9).
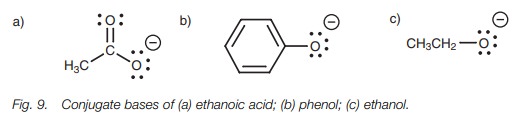
The charge of the carboxylate ion is on an
oxygen atom, and since oxygen is electronegative, the charge is stabilized.
However, the charge can be shared with the other oxygen leading to
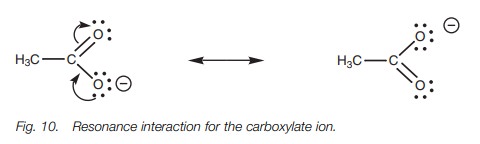
delocalization of the charge. This arises by a resonance interaction between a
lone pair of electrons on the negatively charged oxygen and the π electrons of the carbonyl group (Fig. 10). A lone pair of electrons on the ‘bottom’ oxygen forms a
new π bond to the neighboring carbon. At the same
time as this takes place, the weak π bond of the carbonyl group breaks. This is
essential or else the carbonyl carbon would end up with five bonds and that is
not permit-ted. Both electrons in the original π bond now end up on the ‘top’ oxygen which means that this oxygen
ends up with three lone pairs and gains a negative charge. Note that the π bond and the charge have effectively ‘swapped places’. Both the
structures involved are called resonance structures and are easily
interconvertible. The negative charge is now shared or delocalized equally
between both oxygens and is stabilized. Therefore, ethanoic acid is a stronger
acid than one would expect based on the electronegativity of oxygen alone.
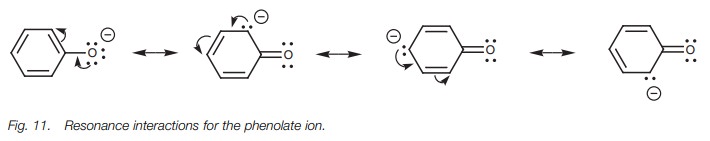
Phenol is less acidic than ethanoic acid but is
more acidic than ethanol. Once again, resonance can explain these differences.
The conjugate base of phenol is called the phenolate ion. In this case, the
resonance process can be carried out sev-eral times to place the negative
charge on four separate atoms – the oxygen atom and three of the aromatic
carbon atoms (Fig. 11). The fact that
the negative charge can be spread over four atoms might suggest that the
phenolate anion should be more stable than the carboxylate anion, since the
charge is spread over more atoms. However, with the phenolate ion, three of the
resonance structures place the charge on a carbon atom which is much less
electronegative than an oxygen atom. These resonance structures will be far
less important than the resonance structure having the charge on oxygen. As a
result, delocalization is weaker for the phenolate ion than it is for the
ethanoate ion. Nevertheless, a certain amount of delocalization still takes
place which is why a phenolate ion is more stable than an ethoxide ion.
Lastly, we turn to ethanol. The conjugate base
is the ethoxide ion which cannot be stabilized by delocalizing the charge,
since resonance is not possible. There is no π bond available to participate in resonance. Therefore, the
negative charge is localized on the oxygen. Furthermore, the inductive donating
effect of the neigh-boring alkyl group (ethyl) enhances the charge and
destabilizes it (Fig. 12). This makes
the ethoxide ion the least stable (or most reactive) of the three anions we
have studied. As a result, ethanol is the weakest acid.

Amines and amides
Amines and amides are very weak acids and only
react with very strong bases.
The pKa
values for ethanamide and ethylamine are 15 and 40, respectively, which means
that ethanamide has the more acidic proton (Fig.
13). This can be explained by resonance and inductive effects (Fig. 14).
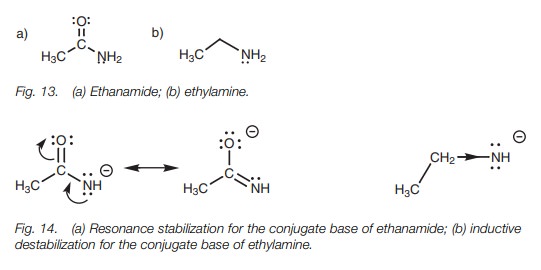
Related Topics